P603 INSPIRE: 6-month interim analysis from an observational post-marketing registry on the effectiveness and safety of darvadstrocel in patients with Crohn’s disease and complex perianal fistulas
Zmora, O.(1);Baumgart, D.C.(2);Faubion, W.(3);Ferrante, M.(4);Gecse, K.(5);Genestin, E.(6);Geransar, P.(6);Hantsbarger, G.(7);Karki, C.(8);Tozer, P.(9);Panés, J.(10);
(1)Shamir Medical Center, Department of Surgery, Tel Aviv, Israel;(2)University of Alberta, Division of Gastroenterology, Edmonton AB, Canada;(3)Mayo Clinic, Gastroenterology and Hepatology, Rochester- MN, United States;(4)University Hospitals Leuven- KU Leuven, Department of Gastroenterology and Hepatology, Leuven, Belgium;(5)Amsterdam UMC, Gastroenterology & Hepatology, Amsterdam, Netherlands Antilles;(6)Takeda pharmaceuticals International AG, Global Medical Affairs - Rare GI, Zurich, Switzerland;(7)Takeda Pharmaceuticals USA Inc., Safety and Health Value Statistics, Cambridge- MA, United States;(8)Takeda Pharmaceuticals USA- Inc., Global Evidence and Outcomes, Cambridge- MA, United States;(9)St Mark’s Hospital and Academic Institute, Fistula Research Unit, London, United Kingdom;(10)Hospital Clinic de Barcelona, IDIBAPS- CIBERehd, Barcelona, Spain;
Background
Darvadstrocel (DVS) is a suspension of expanded allogeneic adipose-derived mesenchymal (stromal) stem cells approved for the treatment of complex Crohn’s perianal fistulas (complex CPF). The INSPIRE (EUPAS24267) registry is a European observational, post-approval study designed to evaluate the real-world effectiveness and safety of DVS in patients with complex CPF for up to 36 months after DVS administration. Here we report clinical and safety outcomes from the first interim analysis at 6 months post treatment.
Methods
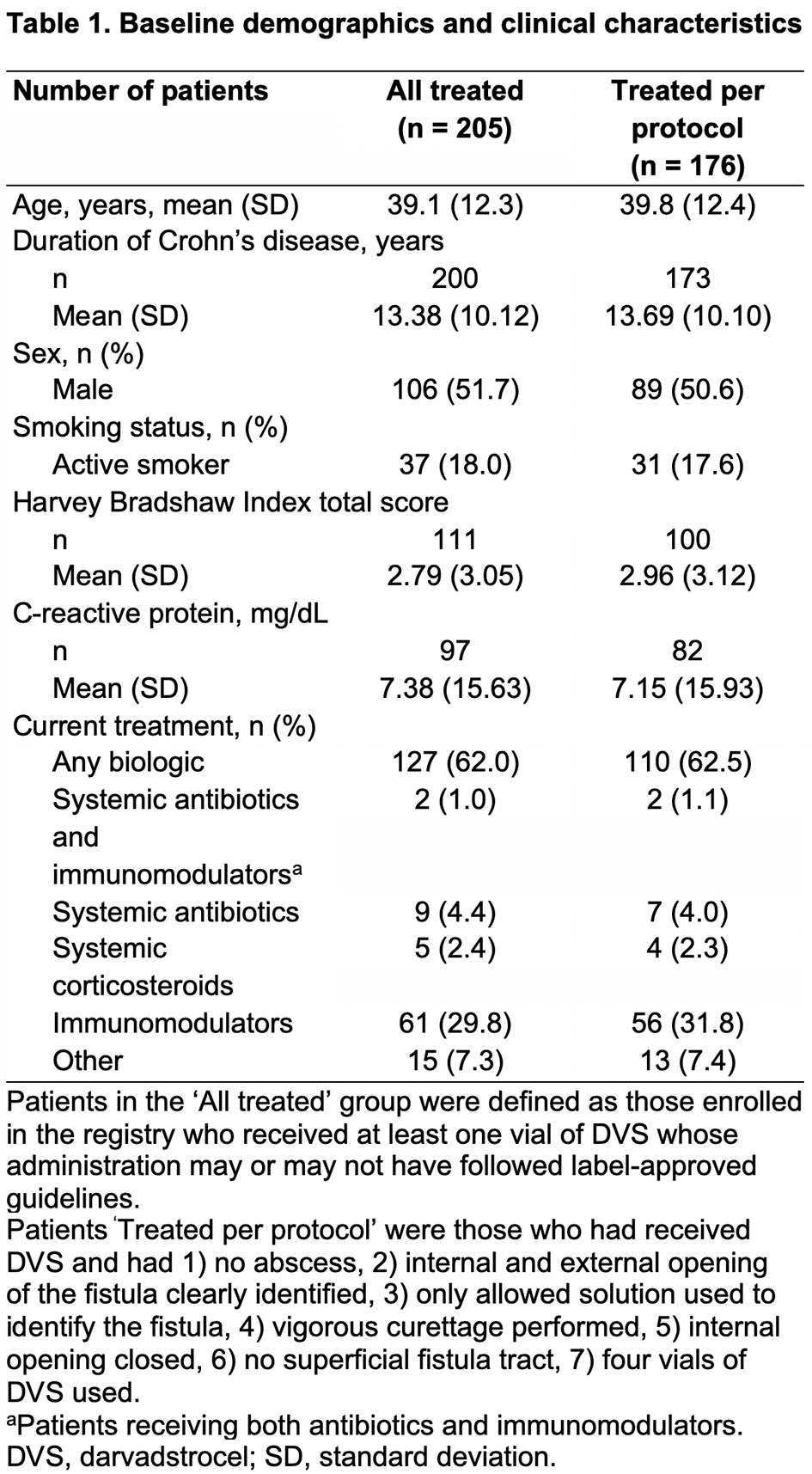
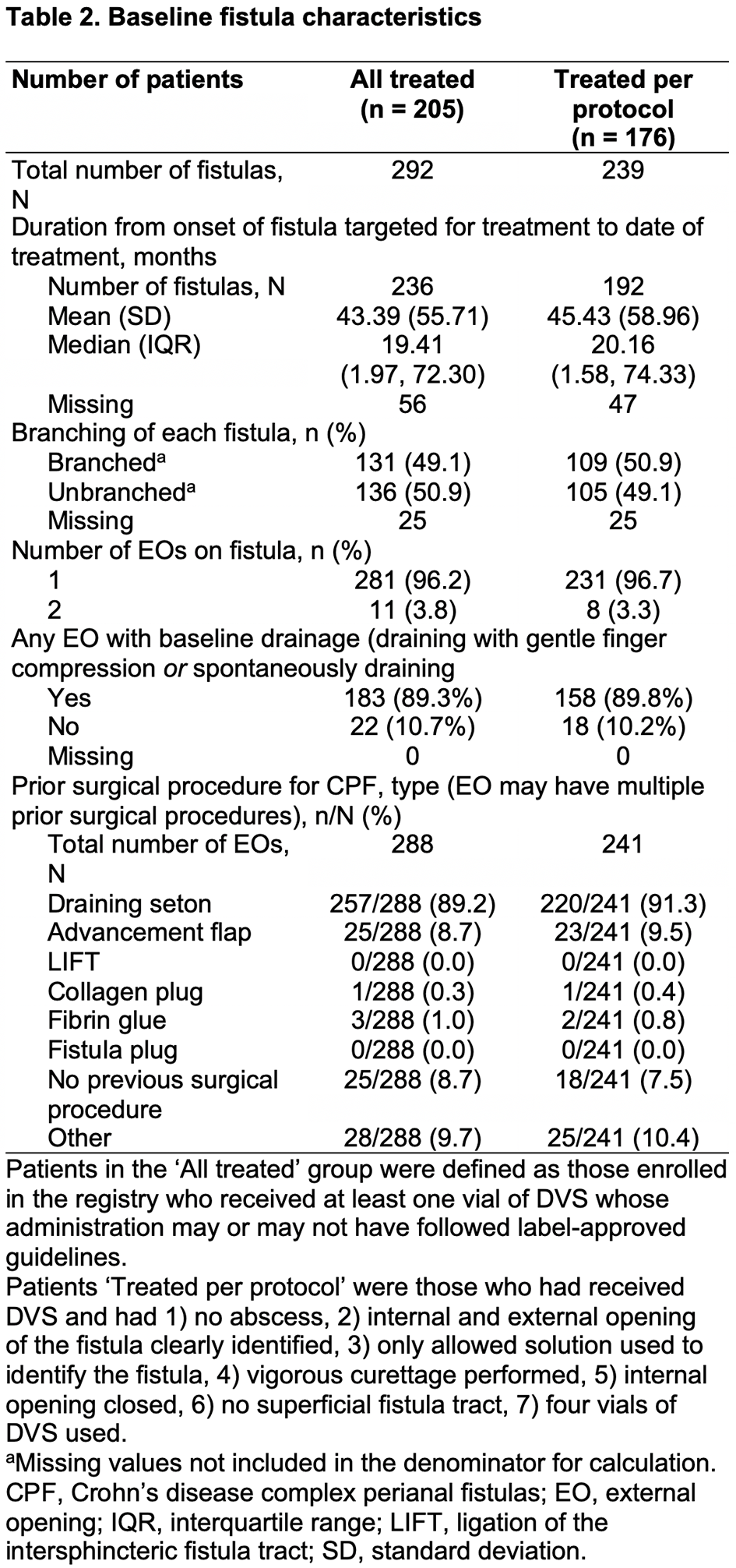
Patients with complex CPF treated with DVS according to local standard practice were eligible to enrol. Primary outcomes were clinical response (defined as closure despite gentle finger compression of ≥50% of external openings treated with DVS that were draining at baseline) and clinical remission (defined as closure despite gentle finger compression of all external openings treated with DVS that were draining at baseline). Outcomes were evaluated in the All Treated (AT) and Treated Per Protocol (PP) cohorts (Table 1) 6 months post treatment in patients who had received a single administration of DVS. The number and nature of adverse events (AEs), serious AEs (SAEs) are reported for the AT cohort.
Results
As of September 2021, 230 patients had enrolled, 225 met the inclusion criteria and 205 had complete treatment data and comprised the AT cohort, of these, 176 were treated per protocol. A total of 138 (AT) and 120 (PP) patients were 6 months post treatment, 66% (92/138) and 58% (69/120) had a 6-month visit, of which, 85% (78/92) and 100% (69/69) had evaluable data at Month 6, respectively. Baseline demographics and clinical and fistula characteristics are shown in Tables 1 and 2. In the AT and PP cohorts, respectively, 73% (57/78) and 74% (51/69) of patients had a clinical response, and 65% (51/78) and 65% (45/69) had clinical remission. Changes in Crohn’s disease (CD) activity (assessed with the Harvey–Bradshaw Index) post treatment were minimal, and most patients had no change in CD activity from baseline. In the AT cohort, 20% (41/205) of patients had ≥1 AE and 9.3% (19/205) had ≥1 SAE. There were no reports of ectopic tissue formation and no deaths.
Conclusion
This is the first real-world, multi-centre study reporting the effectiveness and safety data after DVS treatment for complex CPF. These interim analysis data are consistent with the pivotal ADMIRE-CD study in terms of effectiveness and safety. The ongoing status of the registry and small number of evaluable patients mean these data should be interpreted with caution, pending future analyses in larger numbers of patients with longer follow-up times.


