DOP40 Impact of corticosteroid usage on efficacy and safety outcomes in patients receiving upadacitinib for Ulcerative Colitis
Raine, T.(1);Ishiguro, Y.(2);Rubin, D.(3);Finney-Hayward, T.(4);Dapo, I.(5);Phillips, C.(5);Cheng, E.(6);Targownik, L.(7);Loftus- Jr., E.V.(8);
(1)Addenbrooke's Hospital- Cambridge University Hospitals, Department of Gastroenterology, Cambridge, United Kingdom;(2)Hirosaki General Medical Centre- National Hospital Organisation, Department of Clinical Research, Hirosaki, Japan;(3)The University of Chicago Medicine, Inflammatory Bowel Disease Center, Chicago, United States;(4)AbbVie Ltd., Gastroenterology, Maidenhead, United Kingdom;(5)AbbVie Ltd., Research and Development, North Chicago, United States;(6)AbbVie Ltd., Data & Statistical Science, North Chicago, United States;(7)Mount Sinai Hospital- University of Toronto, Division of Gastroenterology and Hepatology, Toronto, Canada;(8)Mayo Clinic College of Medicine, Division of Gastroenterology and Hepatology, Rochester, United States;
Background
The oral, selective Janus kinase inhibitor upadacitinib (UPA) has demonstrated efficacy as induction and maintenance therapy in patients with moderately to severely active Ulcerative Colitis (UC) in a Phase 3 clinical programme comprising two identical induction trials (U-ACHIEVE Induction [NCT02819635] and U-ACCOMPLISH [NCT03653026]) and a maintenance study (U-ACHIEVE Maintenance). This analysis assessed the impact of baseline corticosteroid (CS) use on the efficacy and safety of UPA in patients in these trials.
Methods
Patients were randomised 2:1 to UPA 45 mg once daily (QD) or placebo (PBO) for 8 weeks. Patients who achieved a clinical response at Week 8 were re-randomised 1:1:1 to UPA 15 mg QD, UPA 30 mg QD or PBO for 52 weeks. Here, we report induction and maintenance endpoints and safety data stratified by baseline CS use.
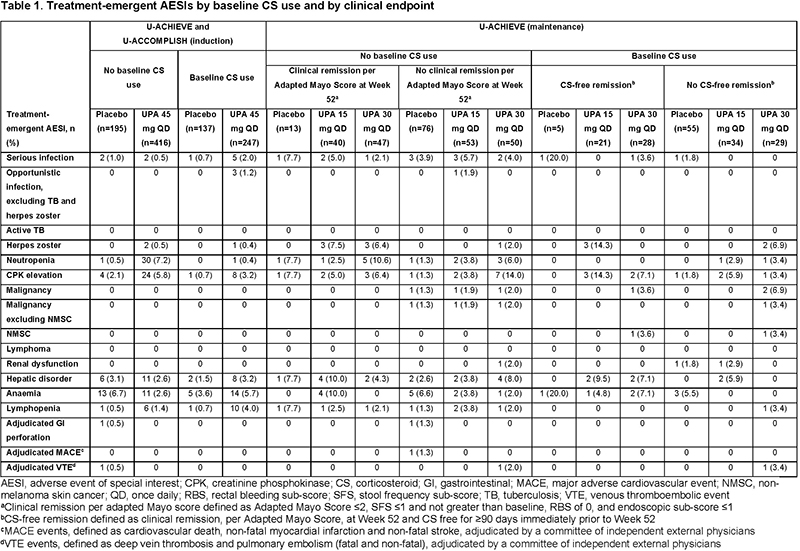
Results
Baseline demographics and disease characteristics were generally well balanced across treatment groups, regardless of baseline CS use. Clinical remission rates among patients receiving UPA 45 mg induction therapy did not differ by baseline CS use (Figure 1A). In the induction period, rates of treatment-emergent adverse events of special interest (AESI), including serious and opportunistic infections, were increased in the UPA 45 mg plus baseline CS group compared with the PBO and UPA without baseline CS groups (Table 1). In the maintenance study, CS tapering was mandated. CS-free remission at Week 52 (defined as clinical remission, per Adapted Mayo Score, and CS free for ≥90 days immediately prior to Week 52) was significantly increased with UPA 30 and 15 mg compared with PBO (both p<0.001; Figure 1B). Among patients receiving UPA maintenance, rates of treatment-emergent AESIs in the baseline CS versus no baseline CS groups were 33% versus 39% and 27% versus 35% in the UPA 30 mg and UPA 15 mg groups, respectively. Malignancy, major adverse cardiovascular events, venous thromboembolic events, and serious and opportunistic infections were reported infrequently in patients receiving UPA (Table 1).
Conclusion
This post hoc analysis suggests that, in patients with moderately to severely active UC, UPA is superior to PBO in conferring CS-free remission. Baseline CS use was not associated with any apparent efficacy benefit and carried a potential increased safety risk. These results suggest that achieving early disease control with UPA and without CS use is an optimal treatment strategy for this population.


