DOP57 Predictive modelling to examine long term treatment effectiveness of darvadstrocel in patients with complex perianal fistulas in Crohn’s Disease
Maruszczak, M.(1);Genenz, K.(2);Turkstra, E.(3);Fenu, E.(2);Hantsbarger, G.(4);Gilaberte, I.(5);Karki, C.(6);Panes, J.(7)
(1)Parexel International, Data Analytics and Machine Learning, Uxbridge, United Kingdom;(2)Takeda Pharmaceuticals, Takeda Pharmaceuticals, Zurich, Switzerland;(3)Parexel International, Parexel Access Consulting, Uxbridge, United Kingdom;(4)Takeda Pharmaceuticals USA- Inc, Statistical and Quantitative Sciences, Cambridge, United States;(5)Takeda Pharmaceuticals USA- Inc, Clinical Sciences, Cambridge, United States;(6)Takeda Pharmaceuticals USA- Inc, Global Evidence and Outcomes, Cambridge, United States;(7)Hospital Clínic de Barcelona- IDIBAPS, Gastroenterology Department, Barcelona, Spain
Background
Darvadstrocel (DVS) was approved in 2018 by EMA for treatment of complex perianal fistulas in Crohn’s disease (CPF-CD)1, with evidence on long-term effectiveness still accruing. The objective of this study was to synthesize clinical trial data and real-world evidence (RWE) using robust statistical methodologies to predict absolute and relative long-term effectiveness of DVS vs. standard of care (SoC) in patients with CPF-CD.
Methods
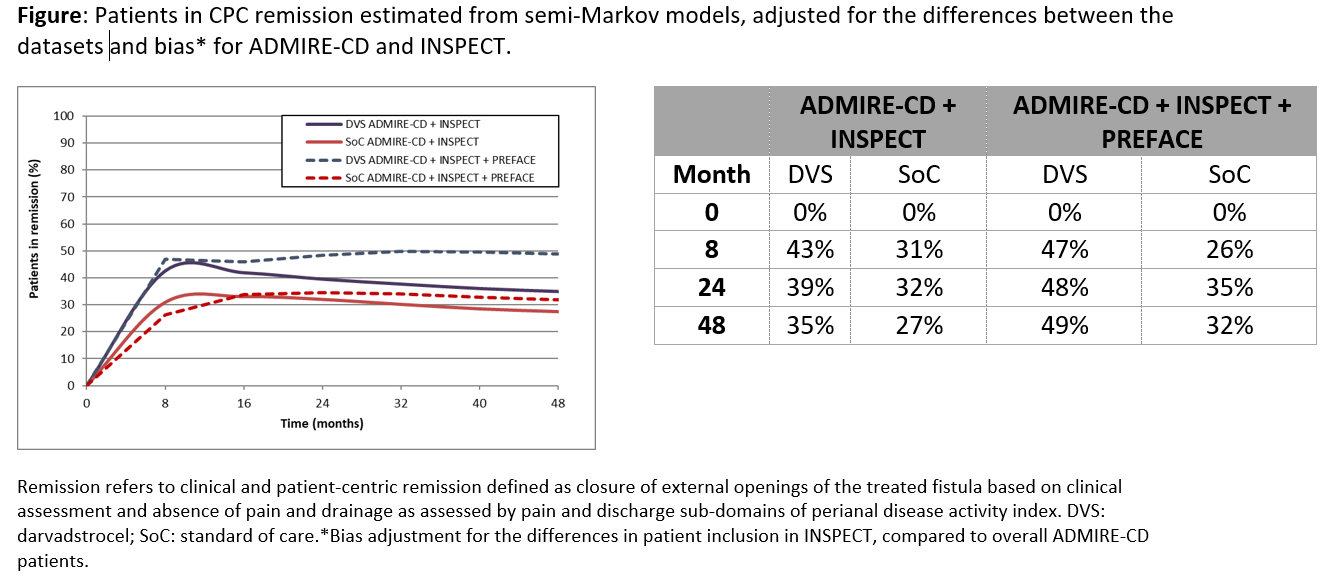
Data was pooled from the pivotal phase 3 trial ADMIRE-CD (n=212; ≤ 2 years data), INSPECT (n=89; chart review study of patients completing ≥52 weeks in ADMIRE-CD, 2-year follow-up post-trial) and PREFACE (n=313; chart review study of patients undergoing SoC treatment, median follow-up of 4 years). Individual anonymized patient data from these studies were combined and analysed with shared covariates of interest included in regression analyses. Weighting procedures were used to adjust for bias due to differences in distribution of outcomes among patients included in INSPECT. Key outcomes were clinical remission, clinical and patient-centric remission (CPC remission), and relapse. Missing values were imputed using a multi-level Bayesian approach. Predictive statistical models extrapolated clinical outcomes beyond the observed follow-up time using parametric curves which were fitted to the defined study populations for each treatment arm and outcome. The models were then implemented within a semi-Markov model, to obtain the resultant number of patients in remission.
Results
Adult patients with CPF-CD from ADMIRE-CD and PREFACE were similar in age, number of internal and external openings of the perianal fistulas. However, the median CD disease duration was 9.5 vs 6.5 years and prior biologic exposure: 79.5% vs 46.0% for patients in ADMIRE-CD and PREFACE respectively. Patients included in INSPECT were comparable to the overall ADMIRE-CD patients for the covariates of interest. Percentage of patients in CPC remission at 24 months was 39% vs 32% for DVS and SoC respectively for ADMIRE-CD+INSPECT. However, after adding PREFACE data to the predictive model and adjusting for outcome bias, the predicted percentage of patients in CPC remission increased in DVS and SoC groups to 48% vs 35%, respectively. There were small differences by 48 months, after which the remission rates stabilized (Figure).
Conclusion
In this pooled analysis, we used predictive modelling to examine the effectiveness of DVS vs. SoC over a longer time. Inclusion of RWE to the model indicates possible maintenance of the disease-modifying effect of DVS over a longer time period.
References: 1. Panés et al. Gastroenterology 2018;154:1334-42
Sponsor: Takeda Pharmaceuticals USA, Inc.