DOP77 Comparative real-world effectiveness and persistence of vedolizumab versus anti-TNF therapy in biologic-naïve patients with Crohn´s Disease with Propensity Score adjustment: Maintenance phase results at week-52 from the prospective VEDOIBD study
Di Giuseppe, R.(1);Plachta-Danielzik, S.(2);Bokemeyer, B.(3);Efken, P.(4);Mohl, W.(5);Hoffstadt, M.(6);Krause, T.(7);Schweitzer, A.(8);Schnoy, E.(9);Atreya, R.(10);Teich, N.(11);Trentmann, L.(12);Ehehalt, R.(13);Franzenburg, S.(2);Hartmann, P.(4);Schreiber, S.(14);
(1)Kompetenznetz Darmerkrankungen, Statistics and Epidemiology, Kiel, Germany;(2)Kompetenznetz Darmerkrankungen, Studienabteilung, Kiel, Germany;(3)Interdisciplinary Crohn Colitis Centre, Crohn Colitis Centre, Minden, Germany;(4)Gastroenterology Practice, Gastroenterology, Minden, Germany;(5)Gastroenterology Practice, Gastroenterology, Saarbrücken, Germany;(6)Gastroenterology Practice, Gastroenterology, Iserlohn, Germany;(7)Gastroenterology Practice, Gastroenterology, Kassel, Germany;(8)Gastroenterology Practice, Gastroenterology, Münster, Germany;(9)University Hospital Augsburg, Department of Gastroenterology, Augsburg, Germany;(10)University Hospital Erlangen- University of Erlangen-Nürnberg Erlangen, Department of Medicine- Medical Clinic 1, Erlangen, Germany;(11)Internistische Gemeinschaftspraxis für Verdauungs- und Stoffwechselkrankheiten, Gastroenterology, Leipzig, Germany;(12)Gastroenterology Practice, Gastroenterology, Bremen, Germany;(13)University Hospital Heidelberg, Department of Gastroenterology, Heidelberg, Germany;(14)University Hospital Schleswig-Holstein- Campus Kiel, Clinic of General Internal Medicine I, Kiel, Germany;
Background
To gain insight into vedolizumab (VDZ) use as a first-line biologic in Crohn´s Disease (CD), this real-world study aimed to assess, within the maintenance phase, the 1-year comparative effectiveness and persistence of VDZ vs anti-TNF therapy in biologic-naïve CD-patients.
Methods
Between 2017-2020, 1200 consecutively enrolled biologic-naïve and biologic-experienced patients with ulcerative colitis (UC) and CD were prospectively included in the VEDOIBD-Registry from 45 IBD-experienced centres across Germany. 294 biologic-naïve CD-patients starting a new therapy with VDZ or anti-TNF (adalimumab: ADA or infliximab: IFX) were included in this real-world evidence (RWE) study. The Kaplan-Meier was used to summarize the treatment persistence from the start of therapy through week-52. The primary outcome was week-52 clinical remission (HBI ≤ 4). Patients were analyzed on a modified intent-to-treat basis (mITT; switchers considered as outcome failure) and on a per-protocol (PP) basis (excluding switchers). To reduce selection bias in the estimation of treatment effects, the inverse probability of treatment weighting propensity score (PS) was implemented. A weighted logistic regression was used to evaluate the effectiveness. The results were reported as odds ratio (OR) and 95% confidence interval (CI).
Results
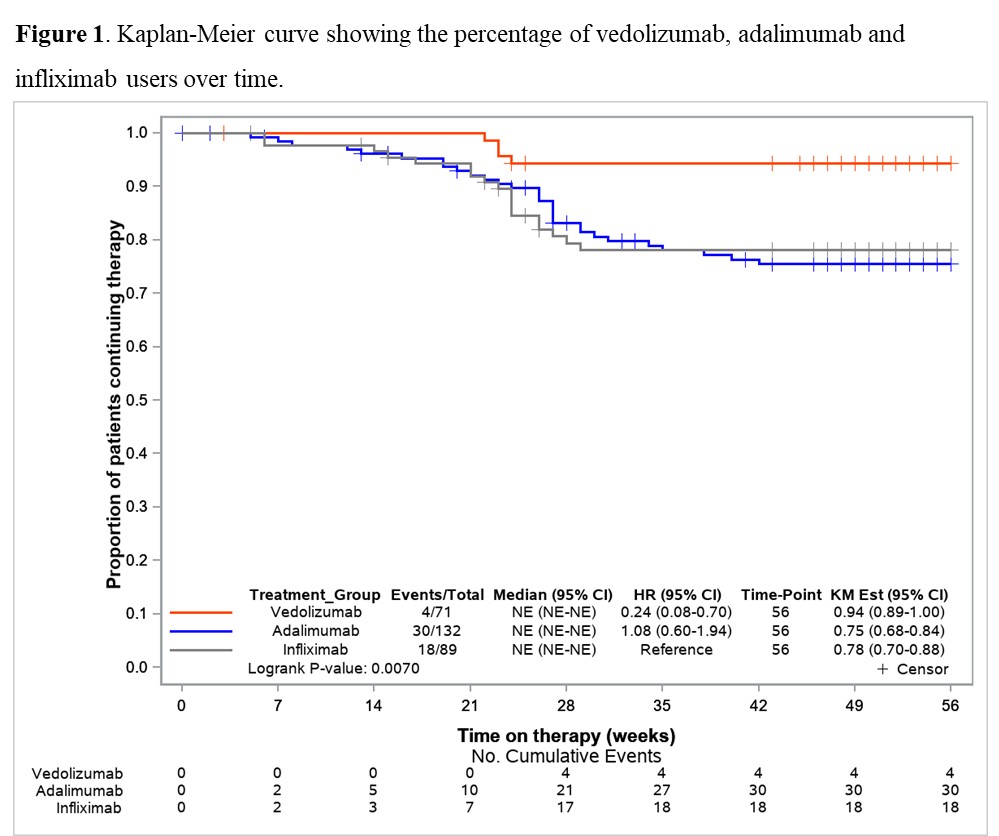
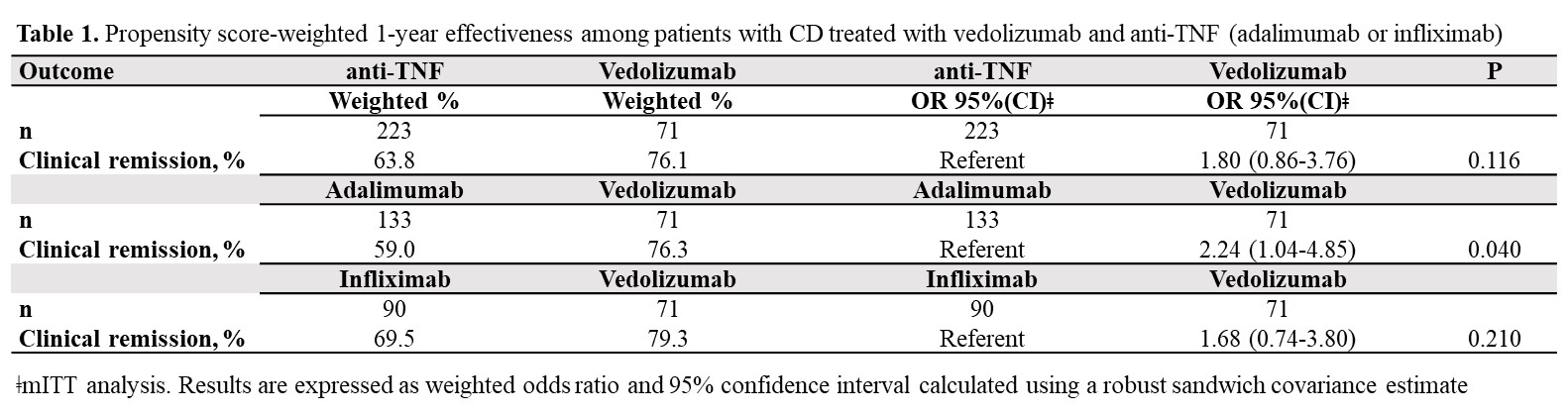
71 VDZ and 223 anti-TNF (ADA: 59.6%, IFX: 40.4%) biologic-naïve CD-patients were evaluated. 52-weeks after treatment initiation approximately 94% of VDZ patients were still in continuous treatment vs 75% of ADA and 78% of IFX (Figure 1). The mITT 1-year clinical remission rate was 76.1% for VDZ vs 63.8% for anti-TNF (OR: 1.80, 95% CI: 0.86-3.76). Similar results were observed for VDZ vs IFX (Table 1). In contrast, the clinical remission was significantly higher in the VDZ group than in the ADA group (OR: 2.24, 95% CI: 1.04-4.85). The PP analysis suggested comparative effectiveness, having excluded more anti-TNF switchers. 91.7% of week-14 responders VDZ patients were in clinical remission from week 14 through 52 vs 66.1% of anti-TNF patients (OR: 5.69, 95% CI: 1.66-19.5). Similar, significant, results were observed for VDZ vs ADA and for VDZ vs IFX (Table 2).
Conclusion
In this real-world setting comparing VDZ and anti-TNF in biologic-naïve patients via PS weighted analysis, VDZ showed especially in week-14 responders higher clinical remission rates in comparison to anti-TNF. The higher treatment persistence observed for VDZ, perhaps due to a more favourable safety profile vs anti-TNF, may be considered the main driver for the better effectiveness of VDZ at one year. These findings may aid physicians’ decision-making on the choice of VDZ as the first-line biologic for CD.