DOP87 The Anti-TL1A Antibody PRA023 Demonstrated Proof-of-Concept in Crohn’s Disease: Phase 2a APOLLO-CD Study Results
Feagan , B.G.(1)*;Sands , B.(2);Siegel , C.A.(3);Dubinsky , M.(4);Longman , R.(5);Sabinho , J.(6);Laurent , O.(7);Luo , A.(7);Lu, JD.(7);Nguyen, D.(7);Towfic , F.(7);DuVall, A.(8);Woyranowski , M.(9);Al Kharrat , H.(10);McGovern, D.P.B.(11);
(1)Department of Medicine, Division of Gastroenterology, Western University, London, ON, Canada;(2)Icahn School of Medicine at Mount Sinai, New York, New York, USA;(3)Dartmouth-Hitchcock Inflammatory Bowel Disease Center, Section of Gastroenterology and Hepatology, Dartmouth-Hitchcock Medical Center, Lebanon, New Hampshire,USA;(4)Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, New York, USA;(5)Division of Gastroenterology, Department of Medicine, Weill Cornell Medical College, New York, NY, USA;(6)Department of Gastroenterology, University Hospitals Leuven, Leuven, Belgium;(7)Prometheus Biosciences, Inc., San Diego, CA, USA;(8)Tyler Research Institute, LLC, Tyler, TX,USA;(9)Department of Pediatrics, Faculty of Medicine and Health Sciences, UJK Kielce, former IP CZD Warsaw, Poland;(10)West Texas Digestive Disease Center, Lubbock, Texas,USA;(11)Karsh Division of Gastroenterology and Hepatology, Department of Medicine, Cedars-Sinai Medical System, Los Angeles, California, USA;
Background
Tumor necrosis factor-like cytokine 1A (TL1A) is an upstream regulator of pro-inflammatory cytokines and fibrosis signals. PRA023 is an anti-TL1A monoclonal antibody in development for multiple inflammatory/fibrotic diseases with a precision medicine approach. This Phase 2a multi-center, open-label study aimed to assess the efficacy and safety of PRA023 as induction treatment in adults with moderately to severely active Crohn’s Disease (CD).
Methods
Key eligibility criteria included Crohn’s disease activity index ≥220 and ≤450 with centrally read [with adjudication] Simple Endoscopic Score (SES)-CD score of ≥6 (≥4 isolated ileal disease) and a history of insufficient/loss of response and/or intolerance to conventional and/or approved biologic therapies (≤4 biologic agents from ≤3 classes). Eligible patients were treated with intravenous PRA023 1000mg on Day 1, 500mg at Weeks 2, 6, and 10. The primary endpoint was endoscopic response (reduction in SES-CD score of ≥50%) at Week 12. Study design utilized historical placebo control rate estimates as a benchmark. Exploratory endpoints include efficacy assessment in a subpopulation identified by a genetics-based companion diagnostic test (CDx).
Results
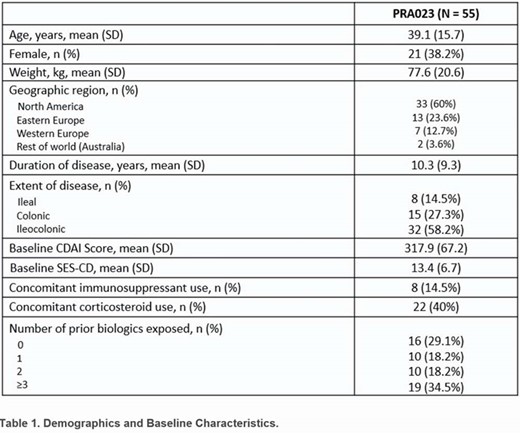
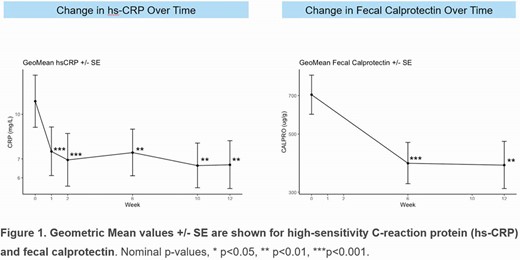
Of the 55 patients in the full analysis data set, 53 (96.4%) completed the 12-week Induction Period. The patient population was notable for the high rate of prior biologic exposure (70.9%) and a mean (SD) disease duration of 10.3 (9.3) years (Table 1). A significantly greater proportion of patients receiving PRA023 achieved endoscopic response (26% PRA023 vs. 12% historical placebo estimate, p=0.002) and clinical remission (CDAI < 150 points; 49% PRA023 vs 16% historical placebo estimate, p< 0.001). Efficacy was observed in biologic-exposed patients (33% endoscopic response and 38.5% clinical remission) while concurrent steroid or immunosuppressant use did not impact efficacy. Significant reductions from baseline in C-reactive protein and fecal calprotectin were observed at all timepoints (Figure 1). Although the pre-specified CDx algorithm provided only limited additional benefit in clinical responses, an alternative CD-specific algorithm (not based on clinical trial data) using the same genetic markers demonstrated enhanced performance across both clinical (12/21 [57%] remission) and endoscopicy response (9/20 [45%]) outcomes. No serious/severe AEs were deemed as study drug-related.
Conclusion
This Phase 2a study demonstrated robust proof-of-concept for PRA023’s efficacy in moderately to severely active CD with favorable tolerability. Patient selection using prespecified genetic markers showed promising results. Placebo-controlled clinical trials will be conducted to confirm these findings.


