N15 A ‘real-world’ observational multi-centre study comparing the side effects and patient acceptability of budesonide MMX with prednisolone in patients with mild to moderate ulcerative colitis
J. Kearns1, L. Scullion1, C. Masterson2, N. Kennedy2, C. Butcher3
1Department Of Gastroenterology, Antrim Area Hospital, Antrim, UK, 2Royal Victoria Hospital, Ambulatory Care Centre, Belfast, UK, 3Department Of Gastroenterology, Causeway Hospital, Coleraine, UK
Background
Budesonide MMX is indicated for the induction of remission in mild to moderate Ulcerative Colitis (UC) patients when 5-ASA treatment is not sufficient. Unlike traditional first-generation glucocorticoid steroids such as prednisolone, budesonide MMX has demonstrated a robust safety profile, comparable to placebo in several randomised controlled trials1,2,3. There is however limited real-world evidence to substantiate this safety claim in clinical practice. The aim of this observational analysis is to evaluate the tolerability and ease of administration of budesonide MMX in the real-world setting using prednisolone as a benchmark.
Methods
Patients receiving treatment for mild to moderate UC were identified in 3 treatment centres between April and October 2019. After providing privacy and data consent, patients completed a detailed nurse-led questionnaire regarding their experiences with prednisolone treatment. Following 6 weeks of therapy with budesonide MMX, patients were sent a follow-up questionnaire. Data from both the initial and subsequent questionnaires were entered by the nurse into a database for assimilation and analysis.
Results
Twenty-eight patients completed initial and follow-up questionnaires. Of these, 78.6% (
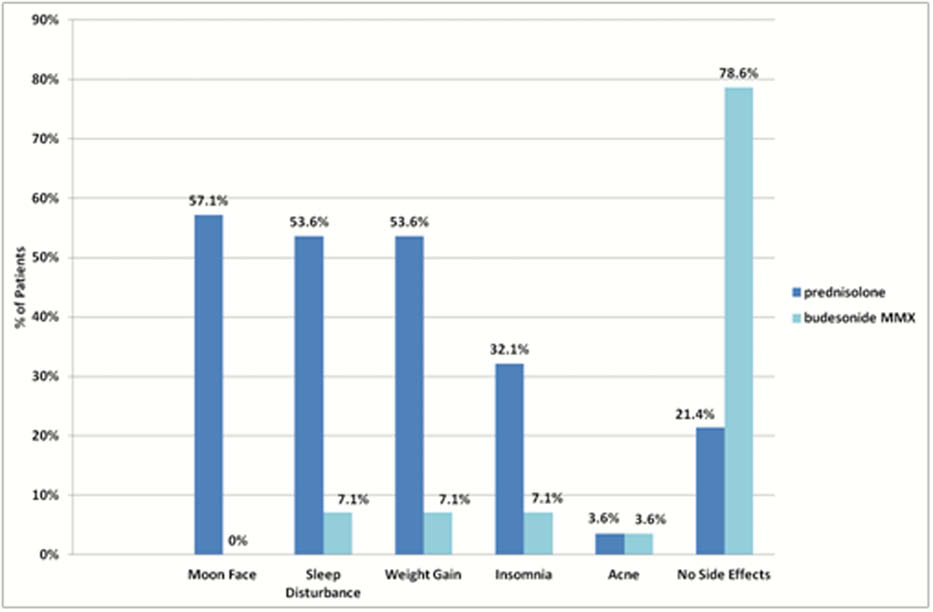
46.4% of patients (
Conclusion
Data from this ‘real-world’ observational study appear to support the safely profile of budesonide MMX reported in clinical trials. The incidence of patients who experienced > 1 side-effect was nearly 4 times lower for budesonide vs. prednisolone. In addition, budesonide MMX therapy was acceptable to the majority of patients and accompanying instructions easy to understand. Additional data will be presented.
Journal of Crohn’s and Colitis, 11(7), pp.785–791.


