OP04 Vedolizumab intravenous is effective across multiple treatment targets in chronic pouchitis: Results of the randomised, double-blind, placebo-controlled EARNEST trial
Travis, S.(1);Silverberg, M.S.(2);Danese, S.(3);Gionchetti, P.(4);Löwenberg, M.(5);Jairath, V.(6,7);Feagan, B.G.(7);Bressler, B.(8);Lindner, D.(9);Escher, A.(9);Jones, S.(9);Shen, B.(10);
(1)John Radcliffe Hospital, Translational Gastroenterology Unit, Oxford, United Kingdom;(2)Mount Sinai Hospital Inflammatory Bowel Disease Centre, Division of Gastroenterology- Department of Medicine, Toronto, Canada;(3)IRCCS Ospedale San Raffaele and University Vita-Salute San Raffaele, Gastroenterology and Endoscopy, Milan, Italy;(4)University of Bologna- S. Orsola- Malpighi Hospital, IBD Unit- Department of Medicine and Surgery DIMEC, Bologna, Italy;(5)Amsterdam University Medical Centre- Location Academic Medical Centre, Department of Gastroenterology and Hepatology, Amsterdam, The Netherlands;(6)Western University, Department of Medicine- Division of Gastroenterology, London, Canada;(7)Alimentiv, Alimentiv, London, Canada;(8)University of British Columbia, Department of Medicine- Division of Gastroenterology, Vancouver, Canada;(9)Takeda Pharmaceuticals International, Takeda Pharmaceuticals International, Zurich, Switzerland;(10)Columbia University Irving Medical Center/Herbert Irving Pavilion, Columbia University Irving Medical Center/Herbert Irving Pavilion, New York, United States;
Background
Pouchitis is a common complication of ileal pouch-anal anastomosis (IPAA) after proctocolectomy in ulcerative colitis (UC). There are currently no approved therapies for chronic pouchitis. Here, we report a multicentre trial of intravenous (IV) vedolizumab (VDZ) for chronic pouchitis after IPAA in patients with UC.
Methods
EARNEST was a randomised, double-blind, placebo (PBO)-controlled, phase 4 study of VDZ in patients aged 18-80 years with chronic pouchitis after proctocolectomy with IPAA for UC (NCT02790138). Male and female patients with a history of IPAA for UC and chronic pouchitis were eligible. Patients were randomised (1:1) to receive VDZ IV (300 mg) or PBO on Day 1 and at Weeks (W) 2, 6, 14, 22 and 30, as well as ciprofloxacin for the first 4 weeks. The primary endpoint was modified Pouchitis Disease Activity Index (mPDAI) remission at W14; efficacy was also assessed through other mPDAI/PDAI secondary endpoints and endoscopic exploratory endpoints (assessed by a central reviewer) at W14 and W34. Safety (adverse events [AEs]) was monitored throughout the study.
Results
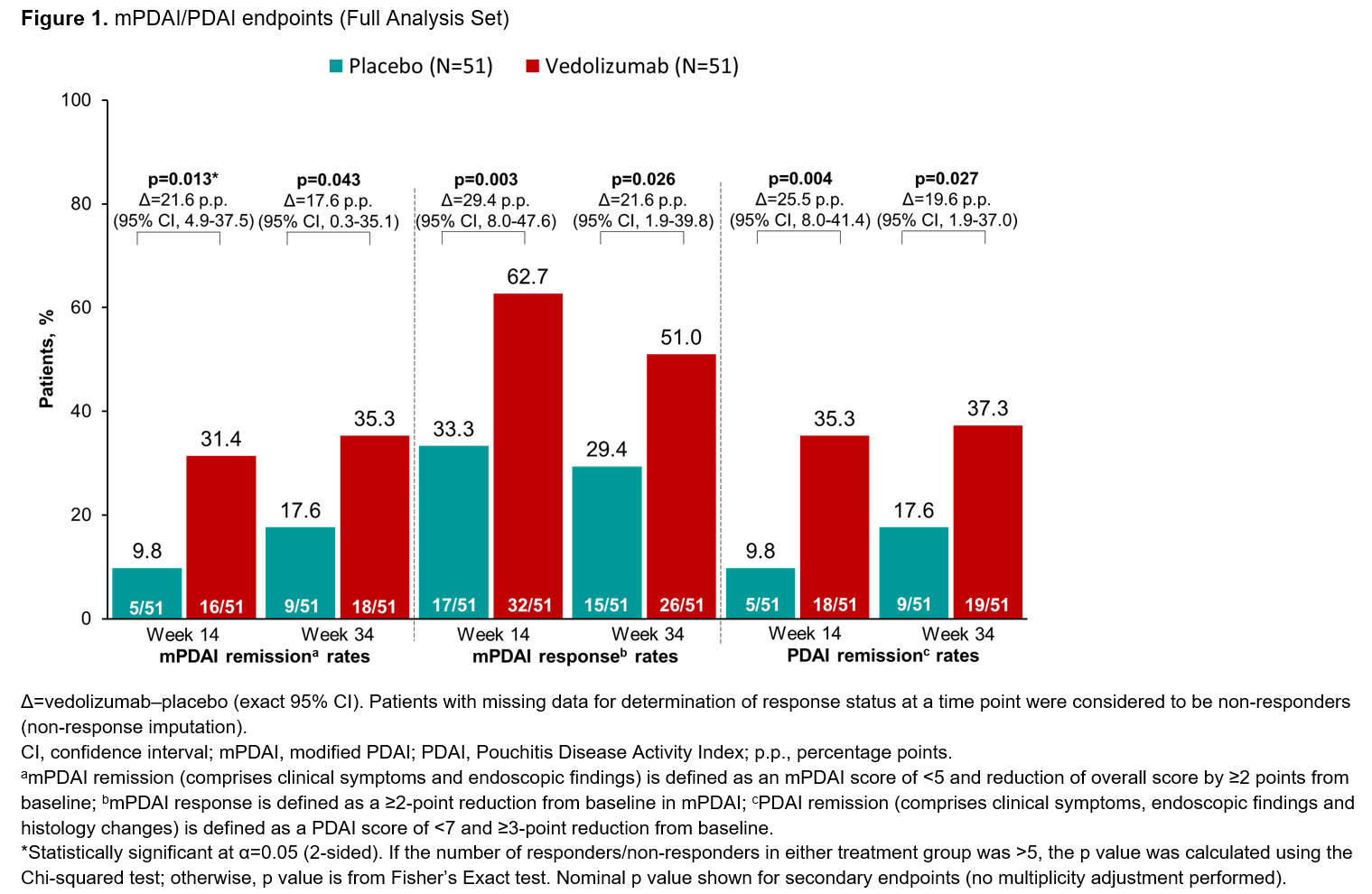
In total, 102 patients were treated (51 per group). Patients had a mean age of 40.8 years (VDZ) and 42.9 years (PBO). mPDAI remission rates (comprising clinical symptoms and endoscopy domains) were 31.4% (n=16/51) for VDZ vs 9.8% (n=5/51) for PBO at W14 (p=0.013; Figure 1). Significant differences in favour of VDZ over PBO were also seen in mPDAI remission at W34, mPDAI response at W14 and W34, and PDAI remission (comprising clinical symptoms, endoscopy and histology domains) at W14 and W34 (Figure 1). The rate of sustained remission (defined as remission at both W14 and W34) was higher for VDZ vs PBO on both the mPDAI (VDZ 27.5% [n=14/51] vs PBO 5.9% [n=3/51]; difference 21.6 percentage points [95% confidence interval (CI), 6.5-37.0]) and the PDAI (VDZ 31.4% [n=16/51] vs PBO 7.8% [4/51]; difference 23.5 percentage points [95% CI, 8.0-38.8]). Endoscopic ulceration analysis showed greater reductions in number of ulcers from baseline for VDZ over PBO at W14 and W34 (Figure 2). A higher proportion of patients in the VDZ vs PBO group had an improved SES-CD score and achieved SES-CD remission of pouchitis (Figure 2). AE rates were similar between groups and no new safety signals were identified (Table).

Conclusion
This is the first and largest randomised, double-blind PBO-controlled trial of biologic therapy to show significant benefits across multiple treatment outcomes in patients with chronic pouchitis after IPAA for UC. VDZ showed consistent treatment benefits over PBO across clinical, endoscopic and histologic endpoints, together with safety consistent with its established profile.