OP07 Exploring disease control by combining clinical, biological, and health-related quality of life remission with endoscopic improvements among Ulcerative Colitis patients treated with filgotinib: A post-hoc analysis from the SELECTION trial
SCHREIBER, S.(1);Feagan, B.(2,3);Peyrin-Biroulet, L.(4);Vermeire, S.(5);Faes, M.(6);Harris, K.(7);Oortwijn, A.(7);Daniele, P.(8);Patel, H.(6);Danese, S.(9,10);
(1)Kiel University, Department of General Internal Medicine, Kiel, Germany;(2)Alimentiv, Inc., London, Canada;(3)London Health Sciences Centre- Western- Western University, Division of Gastroenterology, London, Canada;(4)University Hospital of Nancy- University of Lorraine, Department of Gastroenterology, Vandoeuvre-lès-Nancy, France;(5)University Hospitals Leuven, Department of Gastroenterology and Hepatology, Leuven, Belgium;(6)Galapagos, Nv, Mechelen, Belgium;(7)Galapagos, Nv, Leiden, The Netherlands;(8)Cytel Health, Canada, Toronto, Canada;(9)University Vita-Salute, San Raffaele, Milan, Italy;(10)IRCCS Ospedale San Raffaele, Gastroenterology and Endoscopy, Milan, Italy;
Background
For patients with ulcerative colitis (UC), both subjectively and objectively reported measures are equally important treatment goals. We explored clinical, biological, HRQoL remission and endoscopic improvements as a combined endpoint (CE) from SELECTION (NCT02914522), a phase 2b/3 double-blind trial which assessed the efficacy and safety of filgotinib, a once-daily, oral, Janus 1 kinase preferential inhibitor, for the treatment of UC.
Methods
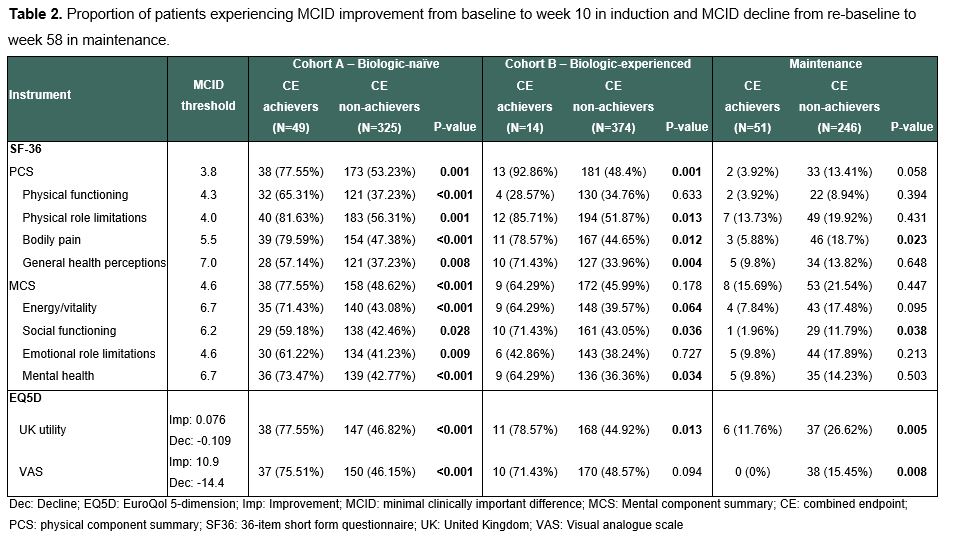
In SELECTION, patients with moderately to severely active UC were randomized 2:2:1 to 200mg filgotinib (FIL200), 100mg filgotinib, or placebo (PBO) for an 11-week induction phase followed by a 47-week maintenance period in patients who achieved clinical remission or response.1 We defined a CE as achieving all of the following: 1) clinical remission defined as partial Mayo Score (excluding endoscopy domain) ≤2 and no sub-score >1), 2) biological remission defined as faecal calprotectin <150 µg/g, 3) Inflammatory Bowel Disease Questionnaire (IBDQ) remission defined as IBDQ >170 and 4) endoscopic improvement defined as Mayo endoscopic sub-score ≤1. We evaluated the CE among patients treated with FIL200 vs placebo in induction (week 10) and maintenance (week 58) phases. Among those achieving the CE, we analysed MCID improvement during induction and decline during maintenance on generic QoL instruments (36-item short-form questionnaire and EuroQol 5-dimension [EQ5D]).2,3,4
Results
The overall population included 381 biologic-naïve and 401 biologic-experienced patients undergoing induction, of which 297 subsequently entered maintenance. A higher proportion of patients receiving FIL200 achieved CE than PBO by week 10 in the biologic-naïve induction cohort (17.6% vs 4.41%, p<0.001) and by week 58 in the maintenance cohort (22.1% vs 7.14%, p=0.002) (Table 1). Biologic-naïve CE achievers had higher MCID improvement across all generic QoL scales and domains (Table 2). Patients achieving CE in maintenance experienced a lower proportion of MCID decline in EQ5D utility, and visual analogue scale.
Conclusion
Treatment with filgotinib resulted in a higher proportion of patients with combined clinical, biological, HRQoL remission and endoscopic improvements. Among patients achieving this combined composite endpoint, clinically meaningful improvements were observed in their overall quality of life. Holistic assessment of several subjective and objective measures may help achieve better outcomes in UC.
1. Feagan BF, et al. Lancet 2021;397:2372–84.
2. User’s manual for the sf-36v2 health survey. Quality Metric, Inc; 2009.
3. Stark RG, et al. Inflamm Bowel Dis. 2010 Jan;16(1):42–51.
4. Irvine EJ. J Pediatr Gastroenterol Nutr. 1999 Apr;28(4):S23–27.