OP23 Efficacy and safety of upadacitinib as induction therapy in patients with Moderately to Severely Active Ulcerative Colitis: Results from phase 3 U-ACCOMPLISH study
Vermeire, S.(1);Danese, S.(2);Zhou, W.(3);Pangan, A.(3);Greenbloom, S.(4);D'Haens, G.(5);Panes, J.(6);Juillerat, P.(7);Lindsay, J.O.(8);Loftus Jr, E.V.(9);Sandborn, W.J.(10);Reinisch, W.(11);Sanchez Gonzalez, Y.(3);Huang, B.(3);Xie, W.(3);Liu, J.(3);Weinreich, M.A.(3);Panaccione, R.(12)
(1)KU Leuven, Gastroenterology, Leuven, Belgium;(2)Instituto Clinico Humanitas, Gastroenterology, Milan, Italy;(3)AbbVie Inc., Research & Development, North Chicago- Illinois, United States;(4)Toronto Digestive Disease Associates, Clinical Research, Ontario, Canada;(5)Amsterdam UMC campus AMC, Gastroenterology, Amsterdam, Netherlands Antilles;(6)Hospital Clínic Barcelona- IDIBAPS- CIBERehd, Inflammatory Bowel Diseases Unit, Barcelona, Spain;(7)Bern University Hospital- Clinic for Visceral Surgery and Medicine, Gastroenterology, Bern, Switzerland;(8)Barts and the London School of Medicine and Dentistry- Queen Mary University of London, Centre for Immunobiology, London, United Kingdom;(9)Mayo Clinic, Division of Gastroenterology and Hepatology, Rochester- Minnesota, United States;(10)University of California San Diego, Division of Gastroenterology, La Jolla- California, United States;(11)Medical University of Vienna, Gastroenterology, Vienna, Austria;(12)University of Calgary, Division of Gastroenterology, Calgary- Alberta, Canada
Background
Upadacitinib (UPA) is a selective and reversible Janus kinase inhibitor.U-ACCOMPLISH is one of two phase 3 induction trials that evaluated the safety and efficacy of UPA 45 mg once daily (QD) in adults with ulcerative colitis (UC).
Methods
U-ACCOMPLISH was a multicentre, randomized, double-blind, placebo-controlled trial (NCT03653026) that enrolled patients with moderate-to-severe UC (defined as adapted Mayo score 5–9 with centrally read endoscopic score 2–3) who had inadequate response, loss of response, or intolerance to aminosalicylates, immunosuppressants, corticosteroids and/or biologics. Patients were randomized 2:1 to UPA 45 mg QD or placebo (PBO) for 8 weeks. At week 8, responders entered the maintenance phase and non-responders entered the extended treatment period to receive open-label UPA 45 mg QD for additional 8 weeks.The primary endpoint (clinical remission per adapted Mayo Score) and ranked secondary endpoints including symptomatic, endoscopic– histologic evaluations from the 8-week PBO-controlled period are reported here. Non-responder imputation incorporating multiple imputation for missing data due to COVID-19 are reported.
Results
522 patients were randomized (UPA, n=345; PBO, n=177); the intent-to-treat population included 341 patients in UPA and 174 patients in PBO group. Baseline demographics and disease characteristics were similar between groups; 50.7% and 51.1% were biologic inadequate responders in UPA and PBO groups, respectively (Table 1). A significantly higher proportion of patients receiving UPA 45 mg QD (33.5%) versus PBO (4.1%) achieved the primary endpoint (adjusted treatment difference: 29.0% [23.2, 34.7]; P<0.001). A significantly higher proportion of patients receiving UPA versus PBO also achieved all ranked secondary endpoints (all P<0.001; Figure 1).Serious adverse events were reported by 3.2% and 4.5% of patients in UPA and PBO groups, respectively (Table 2). Similar rates of serious infection were observed in both groups (0.6%); 2 events each of herpes zoster and opportunistic infection were reported in UPA group. No active tuberculosis, malignancy, adjudicated major adverse cardiovascular events, or deaths were reported in the study. One patient with venous thromboembolism (deep vein thrombosis and pulmonary embolism) and 1 patient with gastrointestinal perforation were reported in the placebo group.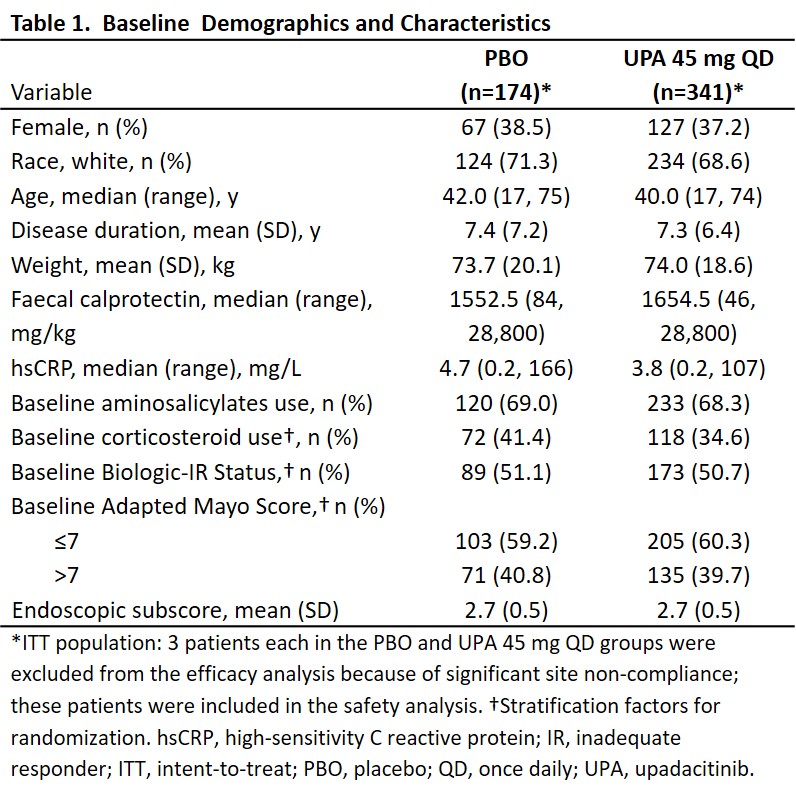


Conclusion
In U-ACCOMPLISH, 8-week UPA 45 mg QD induction treatment led to statistically significant improvements in clinical, endoscopic, and combined endoscopic-histologic endpoints. The treatment was well tolerated, and the safety profile and AE prevalence was comparable with previous studies of UPA with no new safety signals identified.


