OP26 Efficacy and safety of mirikizumab as induction therapy in patients with moderately to severely active Ulcerative Colitis: Results from the Phase 3 LUCENT-1 study
D'Haens, G.(1);Kobayashi, T.(2);Morris, N.(3);Lissoos, T.(3);Hoover, A.(3);Li, X.(3);Arora, V.(3);Milch, C.(3);Sandborn, W.J.(4);Sands, B.E.(5);
(1)Amsterdam University Medical Centers, Gastroenterology, Amsterdam, The Netherlands;(2)Kitasato University Kitasato Institute Hospital, Center for Advanced IBD Research and Treatment, Tokyo, Japan;(3)Eli Lilly and Company, Biomedicines, Indianapolis, United States;(4)University of California San Diego, Gastroenterology, San Diego, United States;(5)Icahn School of Medicine at Mount Sinai, Gastroenterology, New York, United States;
Background
Anti-IL23p19 inhibitors are emerging as promising treatment options for ulcerative colitis (UC). Mirikizumab (miri) is a humanized, IgG4 monoclonal antibody directed against the p19 subunit of IL-23, a key mediator in the pathogenesis of inflammatory bowel diseases. We assessed the induction efficacy and safety of miri with a Phase 3, multi-center, randomized, parallel-arm, double-blind, placebo (PBO)-controlled trial (LUCENT 1; NCT03518086) in patients with moderately to severely active UC (Modified Mayo Score 4-9 points and centrally read Mayo endoscopic subscore ≥2) who had inadequate response, loss of response, or intolerance to corticosteroids, immunosuppressants, biologic therapies, or tofacitinib.
Methods
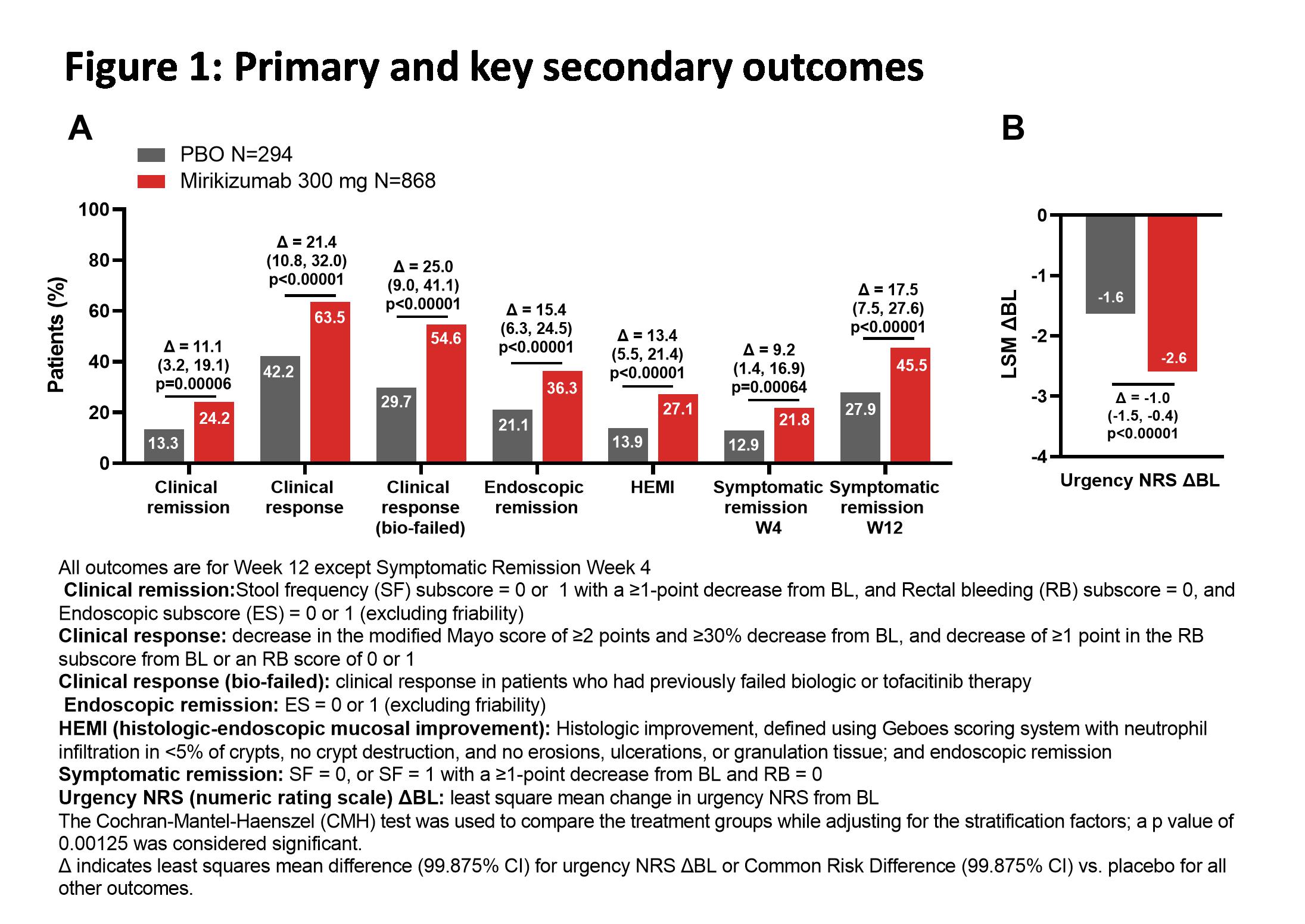
Adult patients (N=1281) were randomized in a 3:1 ratio to receive blinded intravenous administration of 300 mg miri or PBO every 4 weeks for 12 weeks. Randomization was stratified by biologic failure status, baseline (BL) corticosteroid use, BL disease activity as measured by modified Mayo score, and world region. The primary objective was to test the hypothesis that miri was superior to PBO in inducing clinical remission at Week 12. Key secondary objectives were clinical response, endoscopic remission, symptomatic remission, clinical response in biologic-failed patients, histologic-endoscopic mucosal improvement, and improvement in bowel urgency at Week 12 (see Figure for endpoint definitions). Mixed Model for Repeated Measures was used to assess urgency; the Cochran-Mantel-Haenszel test, with missing data imputed as nonresponse, was used to assess all other outcomes.
Results
BL characteristics were balanced across the two treatment groups. A significantly greater proportion of patients treated with miri achieved clinical remission at Week 12 (Miri: 24.2%; PBO: 13.3%; Δ=11.1 [99.875%CI: 3.2, 19.1]; p=0.00006). Miri-treated patients achieved all key secondary endpoints, including a significantly greater average reduction in bowel urgency severity compared to PBO (p<0.00001). The frequencies of treatment-emergent adverse events in miri-treated patients were similar to PBO. There were numerically fewer serious adverse events (Miri: 27 [2.8%], PBO: 17 [5.3%]) and discontinuations due to adverse events (Miri: 15 [1.6%], PBO: 23 [7.2%]) in miri-treated patients compared to PBO. There were 2 colon malignancies in the miri arm (0.2%) and no deaths during the treatment period.
Conclusion
In this phase 3 UC study, 300mg miri IV demonstrated statistically significant and clinically meaningful improvements vs PBO in all primary and key secondary endpoints across clinical, endoscopic, histologic, and symptomatic measures, with an acceptable safety profile.