OP35 Efficacy and safety outcomes up to ~4 years of treatment with filgotinib 200 mg among patients with Ulcerative Colitis: Results from the SELECTIONLTE study
FeaganSenior Scientific Officer, B.G.(1)*;Matsuoka, K.(2);Rogler, G.(3);Faes, M.(4);Oortwijn, A.(5);de Haas, A.(6);Rudolph, C.(5);Peyrin-Biroulet, L.(7);
(1)Alimentiv Inc., Division of Gastroenterology, London, Canada;(2)Toho University Sakura Medical Center, Department of Gastroenterology and Hepatology, Chiba, Japan;(3)University Hospital Zurich, Department of Gastroenterology and Hepatology, Zurich, Sweden;(4)Galapagos NV, Biostatistics Department, Mechelen, Belgium;(5)Galapagos NV, Medical Affairs Department, Leiden, The Netherlands;(6)Galapagos NV, Clinical Development Department, Leiden, The Netherlands;(7)University Hospital of Nancy- University of Lorraine, Department of Gastroenterology, Vandoeuvre-lès-Nancy, France;
Background
Filgotinib (FIL) is a once-daily, oral, Janus kinase 1 preferential inhibitor approved for the treatment of ulcerative colitis (UC). FIL 200 mg (FIL200) was effective in inducing and maintaining clinical remission vs placebo (PBO) and well tolerated in patients with UC in the randomized, phase 2b/3 SELECTION trial (NCT02914522).1 The efficacy and safety of continued FIL200 therapy are being assessed in the continuing SELECTION long-term extension (LTE) study.
Methods
The SELECTION programme comprises SELECTION and the associated LTE study (SELECTIONLTE; NCT02914535). In SELECTION, adults with moderately to severely active UC received induction (IND) FIL200, FIL 100 mg (FIL100) or PBO once daily for 11 weeks. Patients in clinical remission or with a Mayo Clinic Score (MCS) response at week 10 (responders) could enter the 47-week Maintenance (MNT) Study. SELECTIONLTE enrolled patients who completed IND and MNT (completers), patients who were not responders at IND week 10 (non-responders), and patients with disease worsening during MNT. This interim analysis assessed the efficacy and safety of open-label FIL200 through LTE week 144 in completers and LTE week 192 in non-responders, respectively, representing a total of 3.9 years of treatment each (completers: 58 + 144 weeks; non-responders 10 + 192 weeks). The cut-off date was 24 February 2022. Continuous measures of partial MCS (pMCS) and proportions of patients achieving the minimal clinically important difference (MCID) in total IBDQ score (≥16-point increase vs baseline) were assessed without imputation (as observed). Safety was assessed by the exposure-adjusted incidence rate per 100 patient-years of exposure (PYE) for treatment-emergent adverse events (TEAEs) and TEAEs of interest.
Results
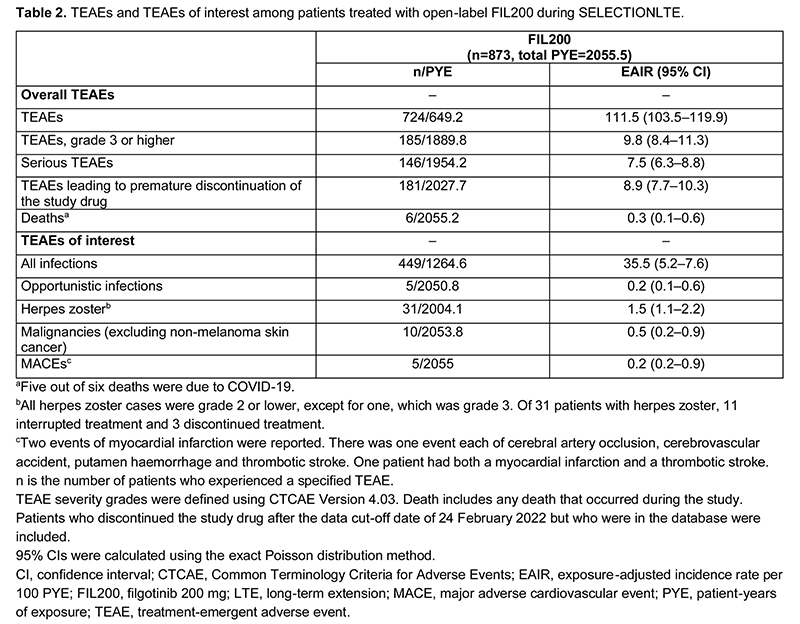
This analysis included 148 completers and 372 non-responders (IND FIL100, n=212; IND FIL200, n=160). Among completers, the reductions in mean pMCS in SELECTION over weeks 0–10 were maintained up to LTE week 144 (Figure 1). In non-responders, mean pMCS decreased from LTE baseline to week 192 (Figure 2). High proportions of completers (>90%) and non-responders (>60%) achieved the MCID in IBDQ score; proportions were maintained over time (Table 1). Safety events among all patients treated with open-label FIL200 in the LTE study (total PYE=2055.5) are reported in Table 2.


Conclusion
FIL200 was effective in maintaining symptomatic remission and health-related quality of life for up to ~4 years. No new safety signals were observed over ~4 years of treatment, and safety events were comparable with those seen in previous analyses.2
1. Feagan BG et al. Lancet 2021;397:2372–84.
2. Feagan BG et al. J Crohns Colitis 2022;16(Suppl1):i456–7.


