P009 IL-3 receptor signalling suppresses chronic intestinal inflammation by controlling mechanobiology and tissue egress of regulatory T cells
Ullrich, K.(1)*;Derdau, J.(1);Baltes, C.(2);Rosso, G.(3);Uderhardt, S.(4);Schulze, L.L.(1);Liu, L.J.(1);Dedden, M.(1);Spocinska, M.(1);Kainka, L.(2);Kubánková, M.(3);Müller, T.M.(1);Schmidt, N.M.(1);Becker, E.(1);Atreya, I.(1);Neurath-Finotto, S.(5);Prots, I.(6);Weigmann, B.(1);López-Posadas, R.(1);Atreya, R.(1);Ekici, A.B.(7);Lautenschläger, F.(8);Guck, J.(3);Neurath, M.F.(1);Zundler, S.(1);
(1)University Hospital Erlangen and Friedrich-Alexander-Universität Erlangen-Nürnberg, Department of Medicine 1, Erlangen, Germany;(2)Saarland University, Experimental Physics, Saarbrücken, Germany;(3)Max Planck Institute for the Science of Light, Max-Planck-Zentrum für Physik und Medizin, Erlangen, Germany;(4)University Hospital Erlangen and Friedrich-Alexander-Universität Erlangen-Nürnberg, Department of Medicine 3, Erlangen, Germany;(5)University Hospital Erlangen and Friedrich-Alexander-Universität Erlangen-Nürnberg, Department of Molecular Pneumology, Erlangen, Germany;(6)University Hospital Erlangen and Friedrich-Alexander-Universität Erlangen-Nürnberg, Department of Stem Cell Biology, Erlangen, Germany;(7)University Hospital Erlangen and Friedrich-Alexander-Universität Erlangen-Nürnberg, Institute of Human Genetics, Erlangen, Germany;(8)Saarland University, Experimental Physics- Center for Biophysics, Saarbrücken, Germany;
Background
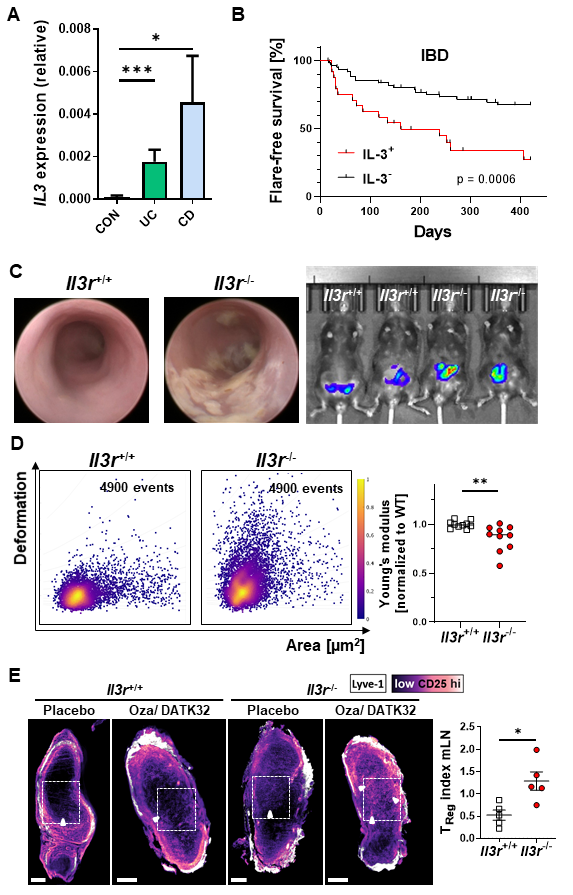
The pathogenesis of inflammatory bowel diseases (IBD) such as Crohn’s disease (CD) and ulcerative colitis (UC) is still incompletely understood. However, despite having been described back in the 1980s and reported to be involved in chronic inflammatory arthritis, the role of interleukin 3 (IL-3) signaling via the IL-3 receptor (IL-3R) in the development and perpetuation of chronic intestinal inflammation remains largely unexplored. In this study, we therefore aimed to explore the role of IL-3 signaling via IL-3R in experimental colitis as well as human IBD.
Methods
We analysed IL-3 expression in lamina propria mononuclear cells (LPMCs) from patients with IBD and from mice with experimental colitis. We compared the development of T cell transfer colitis in Rag1-/- mice after transfer of Il3r-/- and Il3r+/+ naïve T cells and investigated the underlying mechanisms by immunofluorescence and functional cell trafficking assays. The mechanical properties of Il3r-/- and Il3r+/+ T cells were determined by real-time deformability cytometry (RT-DC) and atomic force microscopy (AFM) and cytoskeleton structure was analysed by scanning electron microscopy (SEM) and fluorescence recovery after photobleaching (FRAP).
Results
The expression of IL-3 and IL3+ T cells were increased in the gut of patients with IBD and IL-3 expression was associated to shorter flare-free survival. In vivo, experimental chronic colitis upon T cell transfer was exacerbated in the absence of IL-3 or IL-3R signalling. This was attributable to IL-3-induced changes in kinase signalling and actin cytoskeleton structure, resulting in increased mechanical deformability and enhanced egress of regulatory T (Treg) cells from the inflamed colon mucosa. Similarly, IL-3 controlled mechanobiology in human Treg cells.
Conclusion
We uncover a crucial role of IL-3R signalling in regulating TReg mechanobiology and tissue egress. Together, our data demonstrate a central – probably counter-regulatory – role for IL-3 receptor signalling in Treg cells in the pathogenesis of murine and human chronic intestinal inflammation and therefore suggest that IL-3 might be a novel target for future therapeutic approaches in IBD.


