P016 Dietary supplementation with Brazilian jaboticaba-sabará fruit (Myrciaria jaboticaba (Vell.) O. Berg) ameliorates colitis-driven colorectal cancer in mice
Nascimento, R.D.P.D.(1)*;Rizzato, J.S.(1);Polezi, G.(1);Moya, A.M.T.M.(1);Silva, M.F.(2);Franchi Junior, G.C.(3);Borguini, R.G.(4);Santiago, M.C.P.D.A.(4);Paiotti, A.P.R.(5);Pereira, J.A.(6);Martinez, C.A.R.(6);Marostica Junior, M.R.(1);
(1)University of Campinas, Food Science and Nutrition, Campinas, Brazil;(2)Federal University of São Paulo, Biological Sciences, Diadema, Brazil;(3)University of Campinas, Medical Sciences, Campinas, Brazil;(4)Brazilian Agricultural Research Corporation, Embrapa Food Technology, Rio de Janeiro, Brazil;(5)Federal University of São Paulo, Medicine, São Paulo, Brazil;(6)São Francisco University, Postgraduate Program in Health Sciences, Bragança Paulista, Brazil; Laboratory of Nutrition and Metabolism
Background
Individuals with inflammatory bowel diseases (IBD) are at a high risk of developing colorectal cancer (CRC). Recently, pre-clinical studies have demonstrated that South American crops are rich sources of bioactive compounds that can act against both IBD and CRC. Therefore, our study aimed to characterize the freeze-dried peel of jaboticaba-sabará (Myrciaria jaboticaba (Vell.) O. Berg), a native Brazilian berry, and understand its preventive effects on a mouse model of IBD-related CRC.
Methods
Jaboticaba peel powder was chemically characterized by its contents of polyphenols, carotenoids, and dietary fibers. For the animal experimentation, male BALB/c mice were given in their diets 0, 2.5, or 5% jaboticaba powder for 17 weeks. On day 28, animals received an injection of the carcinogenic azoxymethane. During days 35-42 and 49-56, a solution with dextran sodium sulfate was given as drinking water to mice to induce chronic colitis. Clinical parameters were checked frequently in order to compose the Disease Activity Index (DAI). On day 119, mice were euthanized, their organs were measured, and the colon was checked for visible tumors. Histology processing was performed to assess the incidence of adenocarcinomas and for a score analysis of inflammatory-related parameters.
Results
High-performance liquid chromatography showed that dry jaboticaba is a rich source of cyanidin-3-O-glucoside (1.45%), gallic acid (0.32%), ellagic acid (0.3%), and delphinidin-3-O-glucoside (0.1%). Other compounds were found in a lesser amount, including protocatechuic acid (0.012%), rutin (0.006%), syringic acid (0.005%), and lutein (0.002%). Additionally, the powder showed relevant contents of soluble (11.3%) and insoluble (24.9%) dietary fibers (Table 1). Animals with colitis-driven CRC had diarrhea and showed bleeding in their feces, besides an average of 11 ± 2.1 visible tumors. On the contrary, animals that received 5% jaboticaba had a significant recovery in their colon length and showed reduced signs of colitis, including less bloody diarrhea, therefore, reduced DAI. The 5% intervention decreased the number of tumors to an average of 4.2 ± 1.0 (Figure 1). Adenocarcinoma incidence went from 66.6% in the control cancer group to 37.5% in the 5% jaboticaba group, the latter in which 50% of the animals did not show any alteration (adenoma or adenocarcinoma). Finally, the 5% jaboticaba diet significantly reduced crypt distortion and the depletion of goblet cells. The 2.5% jaboticaba diet had no beneficial effects.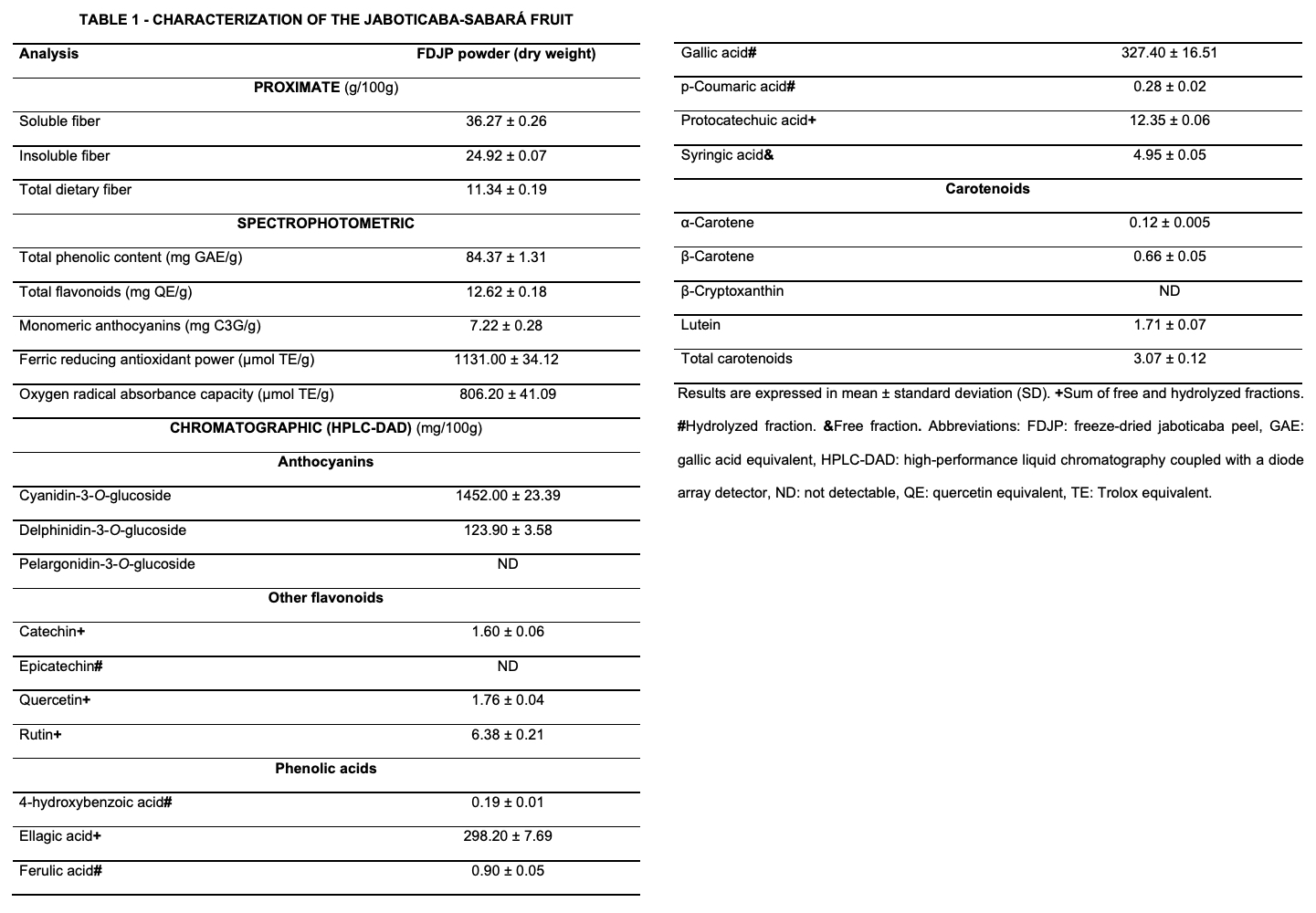

Conclusion
Our preliminary results indicate that anthocyanin- and fiber-rich jaboticaba could represent a potential prevention tool for IBD-related CRC. Further analyses are necessary to understand the molecular mechanisms of jaboticaba on CRC.
- Posted in: Poster Presentations: Basic Science 2023


