P019 Host-Enterobacteriaceae interactions and mitochondrial dysfunction in Crohn’s colitis
Holani, R.(1)*;Bar-Yoseph, H.(2);Krekhno, Z.(3);Moon, K.M.(4);Stacey, R.G.(4);Serapio-Palacios, A.(3);Bressler, B.(5);Donald, K.(6);Foster, L.J.(7);Finlay, B.B.(3);
(1)University of British Columbia, Michael Smith Laboratory- Microbiology and Immunology, Vancouver- British Columbia, Canada;(2)Rambam Health Care Campus, Gastroenterology, Haifa, Israel;(3)University of British Columbia, Michael Smith Laboratory- Microbiology and Immunology, Vancouver, Canada;(4)University of British Columbia, Michael Smith Laboratory- Biochemistry and Molecular Biology, Vancouver, Canada;(5)University of British Columbia, Division of Gastroenterology, Vancouver, Canada;(6)University of British Columbia, Michael Smith Laboratory- Microbiology and Immunolgy, Vancouver, Canada;(7)University of British Columbia, Michael Smith Laboratory- Biochemistry and Molecular Biology, Vanocuver, Canada;
Background
Crohn’s disease (CD) is characterized by chronic gastrointestinal inflammation and unfavourable changes in the composition of intestinal microbiota (‘dysbiosis’). Members of Enterobacteriaceae such as K. pneumoniae and E. coli are often over-represented in colonic mucosa of CD patients. These bacteria contribute to vicious circle of dysbiosis and inflammation by expressing multiple virulence factors. Further, mitochondrial dysfunction in Paneth cells has recently been reported in ileal CD. In agreement, high-fat diet and repeated use of antibiotics increases susceptibility to colitis by damaging mitochondria. However, it remains unknown whether impaired mitochondrial bioenergetics could enhance mucosal colonization by Enterobacteriaceae and thus, contribute to the pathophysiology of colonic CD. Furthermore, the molecular mechanism leading to mitochondrial damage in colonic CD are incompletely understood.
Methods
Right colonic biopsies were collected from 74 adult CD patients and 28 healthy participants at St. Paul’s Hospital (Vancouver, BC, Canada). Microbes in colonic biopsies were characterized by 16S rDNA sequencing using Illumina MiSeq platform, followed by analysis of differentially abundant families via ANCOM-BC methodology. Further, high-throughput data-independent acquisition (DIA) proteomics was performed in colonic biopsies (Bruker Daltonics TimsTof Pro2 LC-MS/MS), and later analyzed via DIA-neural network software. Spearman's rank correlation approach was used for host proteomics- Enterobacteriaceae correlational analysis. Microbiome and proteomic data were corrected for false discovery rate (FDR; < 0.5 for statistical significance). For validation studies, human colonic TC7 epithelial cells and patient-derived organoid cultures were infected with CD-patient derived E. coli ± C75, a chemical inhibitor of fatty acid synthase (FASN; de novo fatty acid synthesis enzyme).
Results
We found an increased abundance of Enterobacteriaceae members in colonic mucosa of CD patients compared to healthy controls (Fig A). Next, our proteomics data revealed a significant downregulation in a group of proteins which were functionally related to mitochondrial health and activity, in CD patients compared to controls (Fig B). Further, Spearman’s analysis showed a positive correlation between Enterobacteriaceae and host FASN enzyme (Fig C), a protein directly related to mitochondrial metabolism. Interestingly, we found that blocking FASN with chemical inhibitor (C75) significantly enhanced colonization by E. coli in TC7 cells (Fig D).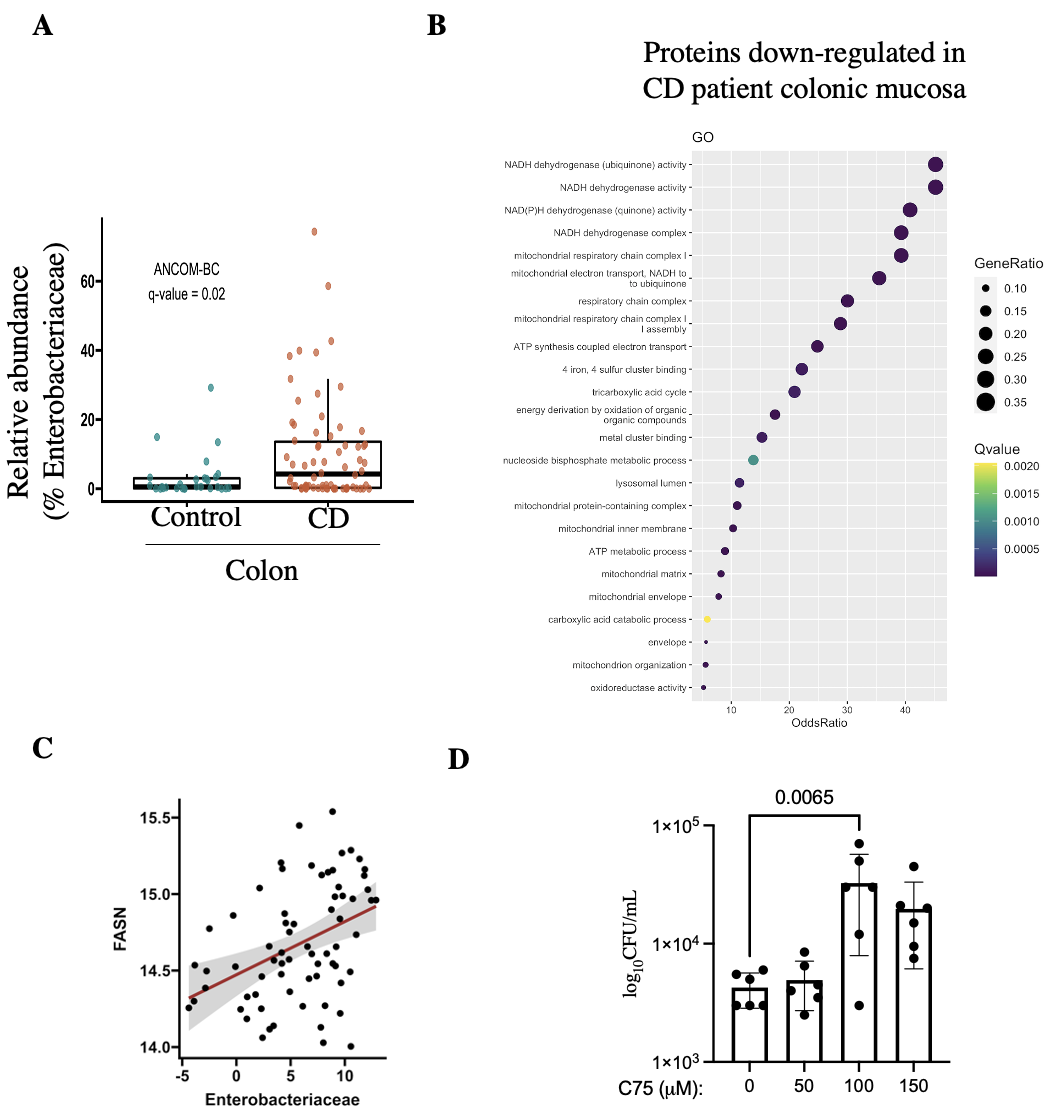
Conclusion
Mechanistically, our study reports that aberrant activity of host FASN enzyme may cause mitochondrial dysfunction and thus, facilitates intestinal colonization by Enterobacteriaceae in CD patients.


