P034 A common IL2RA polymorphism alters natural killer cell immunophenotype and function in Crohn’s disease, with implications for IL-2 based therapy
Clough, J.(1)*;Roberts, L.(2);Goldberg, R.(2);Lord, G.(3);Irving, P.(1);
(1)Guy's and St Thomas' NHS Trust, Gastroenterology, LONDON, United Kingdom;(2)King's College London, School of Immunology and Microbial Sciences, London, United Kingdom;(3)University of Manchester, Department of Medicine and Life Sciences, Manchester, United Kingdom;
Background
The single nucleotide polymorphism (SNP) rs61839660 lies within the IL2RA (CD25) intronic enhancer and has been identified as a causal variant in Crohn’s disease (CD), with a heterozygote frequency of 16.8% in a European population. CD25 is the high-affinity receptor for IL-2, and is constitutively expressed on regulatory T cells (Tregs). Low-dose IL-2 (LD-IL2) is under investigation in IBD, under the premise that it will expand a group of tolerising Tregs. However, finding a therapeutic dose which activates Tregs without activating effector T cells (Teffs) has proved challenging and activation of CD56dim NK cells has been linked to adverse events in trial subjects. We have demonstrated that the rs61839660 risk (T) allele carriers exhibit higher CD4+ T cell CD25 expression, enhanced pSTAT5 activation on stimulation with IL-2, and greater Teff release of pro-inflammatory cytokines. We sought to understand the impact of the risk allele on NK cell immunophenotype and function.
Methods
We recruited CD patients homozygous major allele (CC), or heterozygous (CT) at the rs61839660 locus. We extracted peripheral blood mononuclear cells and flow-sorted CD56dim (cytotoxic) and CD56bright (regulatory) NK subsets. Cells were stimulated with IL-2, then stained for intracellular pSTAT5. To assess cytotoxicity, NK cells were rested in media either with or without IL-2, before plating with CFSE-labelled K562 target cells. The proportion of K562 cells positive for dead cell marker was assessed for each condition.
Results
There was no difference in CD phenotype or severity between groups, in terms of age at diagnosis, sex distribution, presence of perianal disease or the proportion requiring surgery. CD25 expression was significantly enhanced on CD56Bright NK cells in CT subjects compared to CC subjects (1b). Enhanced CD25 expression was also seen in CD56Dim NK cells, though this did not reach statistical significance (1a). The concentration of IL-2 required to activate 50% intracellular STAT5 was lower in CT subjects’ CD56Bright NK cells compared to those from CC subjects (21.5IU/ml vs. 132.4IU/ml, p=0.039). The cytotoxicity of CT subjects’ CD56dim NK cells was significantly enhanced following exposure to IL-2, compared to CC subjects (1c).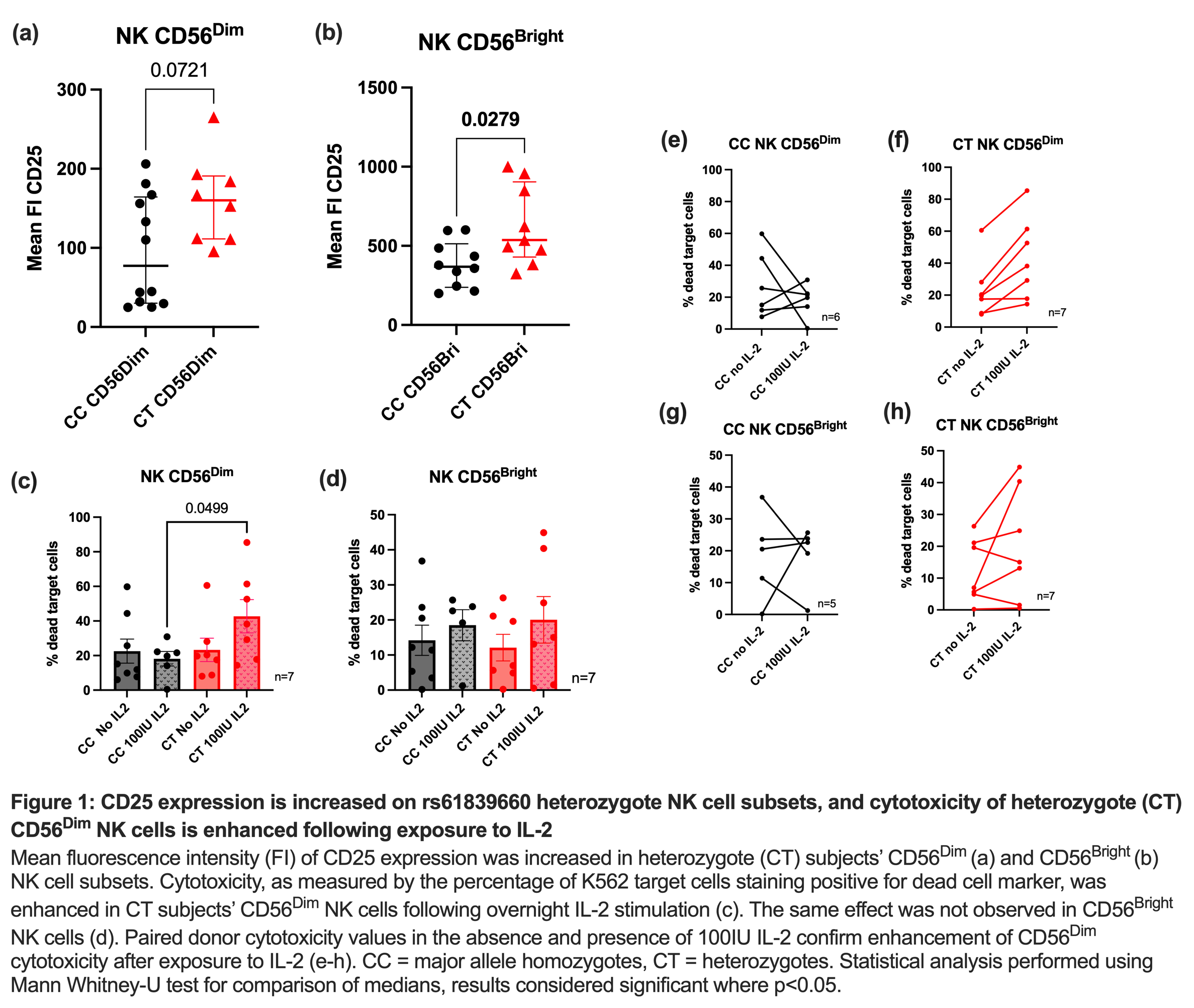
Conclusion
Our findings suggest that risk (T) allele carriers have enhanced responsiveness to IL-2 signalling in NK cells, mediated by increased expression of CD25. We hypothesise that these subjects would be at greater risk of adverse events on exposure to LD-IL2, through enhanced CD56Dim NK cytotoxicity. Our results suggest that screening of subjects entering trials of IL-2-based therapy for the rs61839660 risk variant should be considered, as the threshold for adverse events in these subjects may be lower.


