P041 The upregulation of tissue factor in colonic mesenchymal cells in active ulcerative colitis is associated with severe and resistant to treatment disease, but tofacitinib has not effect on it
Drygiannakis, I.(1)*;Tzenaki, N.(1);Archontoulaki, E.(2);Filidou, E.(3);Kandilogiannakis, L.(3);Valatas, V.(1);Kolios, G.(3);Koutroubakis, I.E.(1);
(1)University of Crete, Gastroenterology Research Laboratory- School of Medicine, Heraklion, Greece;(2)Democritus University of Thrace, Department of Molecular Biology and Genetics- School of Health Sciences, Alexandroupolis, Greece;(3)Democritus University of Thrace, Laboratory of Pharmacology- Department of Medicine- School of Health Sciences, Alexandroupolis, Greece;
Background
Tissue factor (TF) plays an important role on blood clotting and the risk of thromboembolic events is increased in ulcerative colitis (UC). Tofacitinib, recently introduced in UC therapeutics, may further increase the risk.
To delineate thrombosis pathophysiology in this context, TF expression in primary human colonic mesenchymal cells (PHCMC) of patients with active UC was correlated with clinical parameters, serum markers of inflammation and endoscopy. We then treated those PHCMC with tofacitinib with or without cytokines to better mimic the in vivo milieu they are exposed to.
Methods
PHCMC from endoscopic biopsies of the inflamed mucosa of 10 UC patients with an endoscopic Mayo score ≥2 were treated with all major T helper (Th)1 (TNF-α, IFN-γ), Th2 (IL-4, IL-13) or T regulatory (Treg; TGF-β, IL-10) cytokines with or without tofacitinib. Cells were lysed, RNA was isolated and reverse-transcribed to cDNA. TF and RPL4 (housekeeping) cDNAs were quantified with real-time PCR. Wilcoxon and Mann-Whitney U tests were used to compare TF ΔΔCT for paired or unpaired values, respectively, and Spearman’s rho to correlate TF ΔΔCT with scale clinical variables and continuous laboratory values.
Results
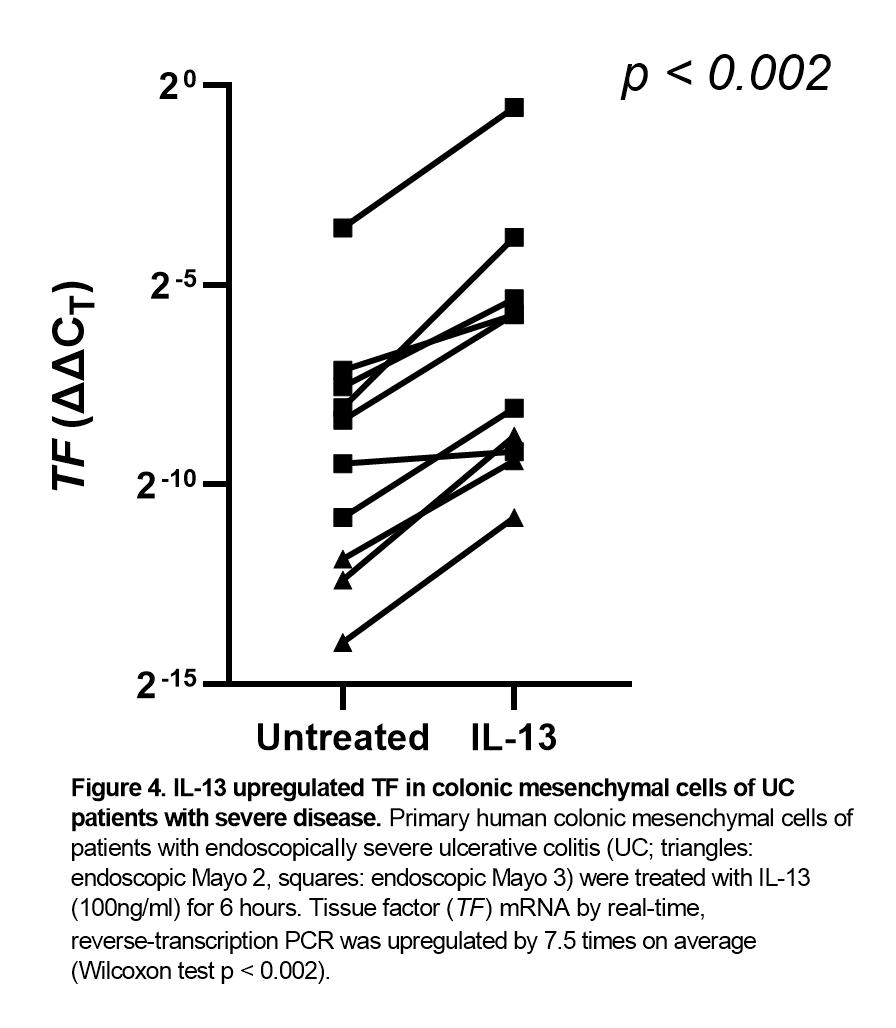
Increased TF mRNA abundance in PHCMC was correlated to increased partial Mayo score (Spearman's rho 0.661, p < 0.044), reduced serum albumin (Spearman's rho -0.723, p < 0.05) and to more severe endoscopic lesions: cells originating from UC patients of an endoscopic Mayo score 3 expressed 20 times more TF than those from an endoscopic Mayo score 2 (p < 0.017; Figure 1). Moreover, PHCMC from difficult-to-treat patients, defined as requiring >1 biologics, expressed 9 times more TF (Figure 2).
On the other hand, treatment with tofacitinib did not upregulate TF (Figure 3). PHCMC expressed receptors and responded to treatment with all major Th1 (TNF-α, IFN-γ), Th2 (IL-4, IL-13) or Treg (TGF-β, IL-10) cytokines by further upregulating TF by 5.5-7.5 times (p 0.001-0.035). IL-13 had the maximal effect (Figure 4). Even when tofacitinib was added together with the aforementioned cytokines, it did not further upregulate TF; instead, it tended to partially inhibit their effects. For example, it decreased upregulation of TF by IL-13 by 40%.
Conclusion
The expression of TF mRNA in active UC is significantly associated with clinically and endoscopically severe disease and resistance to treatment. Tofacitinib per se does not increase TF. Instead, it may limit the upregulating effect of pro-inflammatory cytokines.