P049 Proteomic characterization of serum extracellular vesicles from newly diagnosed patients with inflammatory bowel disease
Soleto, I.(1)*;Baldan, M.(1);Ramírez, C.(1);Orejudo, M.(1);García, S.(1);Mercado, J.(1);Azkargorta, M.(2);Lloro, I.(2);Ortega Moreno, L.(3);Aldars Garcia, L.(1);Riestra, S.(4);Rivero, M.(5);Gutiérrez, A.(6);Rodríguez-Lago, I.(7);Fernández, L.(8);Ceballos, D.(9);Benítez, J.M.(10);Aguas, M.(11);Bastón Rey, I.(12);Bermejo, F.(13);Casanova, M.J.(1);Lorente, R.(14);Ber, Y.(15);Ginard, D.(16);Esteve, M.(17);Elortza, F.(2);Gisbert, J.P.(1);Martin-Cofreces, N.(18);Chaparro, M.(1);
(1)Hospital Universitario de La Princesa- Instituto de Investigación Sanitaria Princesa IIS-Princesa- Universidad Autónoma de Madrid UAM- and Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas CIBERehd, Gastroenterology Unit, Madrid, Spain;(2)Proteomics Platform- CIC bioGUNE- BRTA Basque Research & Technology Alliance- CIBERehd, Proteomics Platform, Derio, Spain;(3)Área de Farmacología y Nutrición y Bromatología. Departamento Ciencias Básicas de la Salud. Universidad Rey Juan Carlos, Gastroenterology Unit, Madrid, Spain;(4)Hospital Universitario Central de Asturias and Instituto de Investigación sanitaria del Principado de Asturias ISPA, Gastroenterology Unit, Asturias, Spain;(5)Hospital Universitario Marqués de Valdecilla and IDIVAL, Gastroenterology Unit, Santander, Spain;(6)Hospital General Universitario de Alicante, Gastroenterology Unit, Alicante, Spain;(7)Hospital Galdakao-Usansolo, Gastroenterology Unit, Vizcaya, Spain;(8)Hospital Clínico Universitario de Valladolid, Gastroenterology Unit, Valladolid, Spain;(9)Hospital Universitario de Gran Canaria Dr. Negrín, Gastroenterology Unit, Las Palmas de Gran Canaria, Spain;(10)Hospital Universitario Reina Sofía and IMIBIC, Gastroenterology Unit, Córdoba, Spain;(11)Hospital Universitari i Politecnic La Fe and Health Research Institute IISLaFe, Gastroenterology Unit, Valencia, Spain;(12)Complexo Hospitalario Universitario de Santiago, Gastroenterology Unit, Santiago de Compostela, Spain;(13)Hospital Universitario de Fuenlabrada, Gastroenterology Unit, Madrid, Spain;(14)Hospital General Universitario de Ciudad Real, Gastroenterology Unit, Ciudad Real, Spain;(15)Hospital San Jorge, Gastroenterology Unit, Huesca, Spain;(16)Hospital Universitari Son Espases, Gastroenterology Unit, Palma de Mallorca, Spain;(17)Hospital Universitari Mutua Terrasa- Terrasa- and Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas CIBERehd, Gastroenterology Unit, Terrasa, Spain;(18)Immunology Unit from Hospital Universitario de La Princesa and Instituto de Investigación Sanitaria Princesa- Universidad Autonoma de Madrid UAM- Centro de Investigación Biomédica en Red Cardiovascular CIBERCV, Immunology Unit, Madrid, Spain;
Background
The etiology and pathogenesis of inflammatory bowel disease (IBD), Crohn's disease (CD), and ulcerative colitis (UC) are complex and the mechanisms that lead to the development of these diseases remain unclear. Extracellular vesicles (EVs) are small particles covered with a cell membrane, originating from the emitting cell, excreted into the extracellular medium, and subsequently captured by receptor cells. EVs have a role in multiple diseases and they could also have a function in IBD pathogenesis. Therefore, the analysis of EVs isolated from the serum of newly diagnosed IBD patients (before starting any treatment) may represent an appropriate experimental approach to elucidate their role in IBD pathogenesis. We aimed to evaluate EVs’ composition and potential effects in the pathogenesis of IBD.
Methods
The protein content of EVs isolated by size exclusion chromatography from the 500 µl of serum from 100 patients with IBD (50 patients with CD and 50 patients with UC) recently diagnosed and 50 healthy controls (HC) was characterized by proteomics and their size and concentration in serum by Nanoparticle tracking analysis (NTA). The biological function of EVs and alterations in signaling pathways related to IBD were determined using Ingenuity Pathways Analysis (IPA) to analyze the OMICs results.
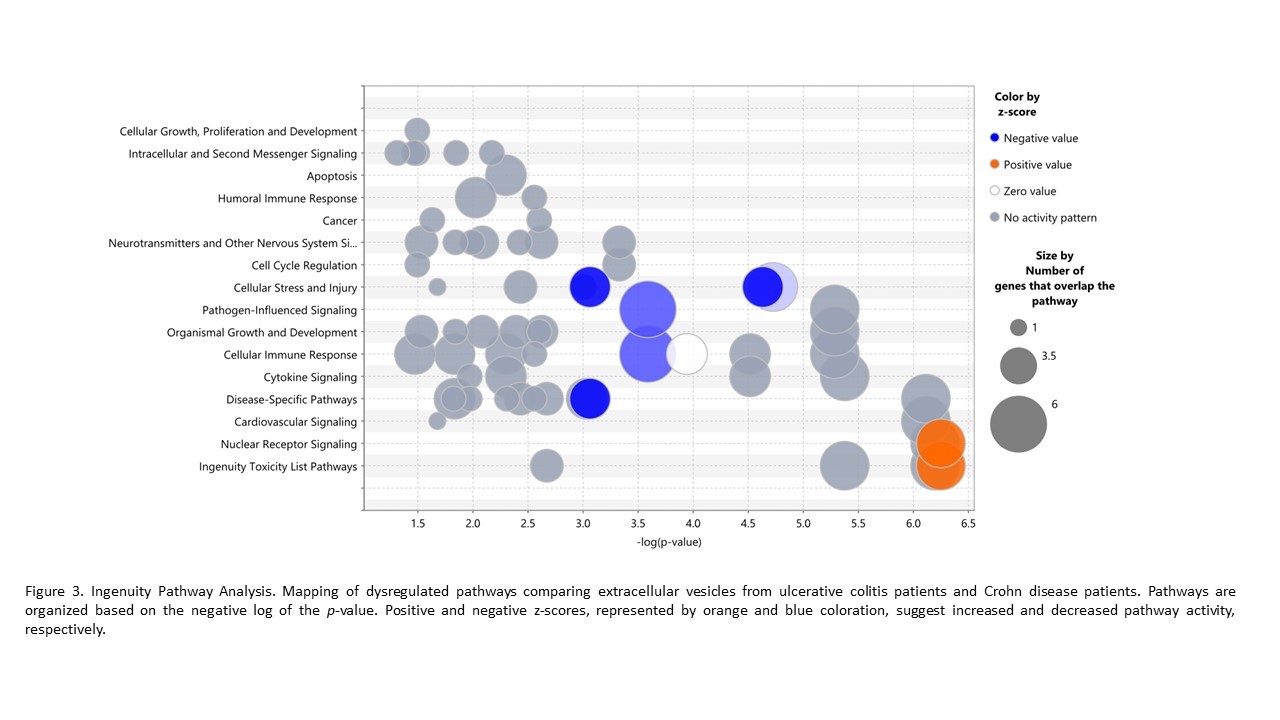
Results
A total of 1,100 proteins have been identified, of which 105 proteins were differentially expressed by patients with CD versus HC, 111 proteins by patients with UC versus HC, and 32 proteins by patients with UC versus CD. IPA analysis revealed that proteins carried in EVs are involved in the dysregulation of immune pathways such as the acute phase response (Figure 1), LXR/RXR activation pathway (Figure 2), and the complement pathway. These pathways were regulated differentially in IBD patients compared with HC, but also when the CU group was compared with the CD group (Figure 3) being upregulated nuclear receptors signaling and cytotoxicity pathways.
Conclusion
EVs carry proteins that can be involved in the dysregulation of the immune system in IBD; this effect would be different in UC and CD since their EVs show a differential profile. Consequently, EVs may play role in IBD pathogenesis and source of strong biomarkers candidates for diagnosis in UC and CD. Further studies on their specific function during IBD are warranted.