P063 The immunological landscape of intestinal fibrosis in Crohn’s Disease.
Jacobs, I.(1,2);Creyns, B.(1,2);Dragoni, G.(2,3);Cremer, J.(1,2);Bislenghi, G.(4);D'Hoore, A.(4);Ke, B.J.(2);Matteoli, G.(2);De Hertogh, G.(5);Ferrante, M.(2,4);Joao, G.S.(2,4);Breynaert, C.(1,6);Vermeire, S.(2,4);Verstockt, B.(2,4);Vanuytsel, T.(2,4);
(1)KU Leuven, Microbiology- Immunology and transplantation, Leuven, Belgium;(2)KU Leuven, Chronic Diseases and Metabolism, Leuven, Belgium;(3)University of Florence, Biomedical Clinical and Experimental Sciences, Florence, Italy;(4)University Hostpitals Leuven, Gastroenterology and Hepatology, Leuven, Belgium;(5)University Hospitals Leuven, Imaging and Pathology, Leuven, Belgium;(6)University Hospitals Leuven, General Internal Medicine, Leuven, Belgium
Background
Patients with Crohn’s disease (CD) often develop strictures, necessitating surgical intervention. The immune pathways expressed in the fibrotic regions, especially in the deeper intestinal layers, are poorly characterized hence hampering therapeutic development for anti-fibrotic agents. We performed a detailed analysis of the immune cell populations in both inflammation and fibrosis in the mucosa and deeper intestinal layers in CD patients.
Methods
Patients with stricturing CD (n=25) or colorectal cancer (CRC, n=10), undergoing ileocolonic resection were included. The resection specimen of CD patients was macroscopically subdivided into fibrotic, inflammatory but not fibrotic, and unaffected regions by an IBD-experienced histopathologist. The mucosal layer was furthermore separated from the deeper intestinal layers. Immune cells were isolated, followed by flow cytometry. Quantitative real time PCR (qRT-PCR) was performed for the eosinophil chemoattractant CCL11 coding for eotaxin-1 and normalized against the housekeeping genes RPL13a and PPIA. Wilcoxon matched-pairs sign rank test was performed on paired samples while Dunn’s multiple testing was performed for unpaired comparisons.
Results
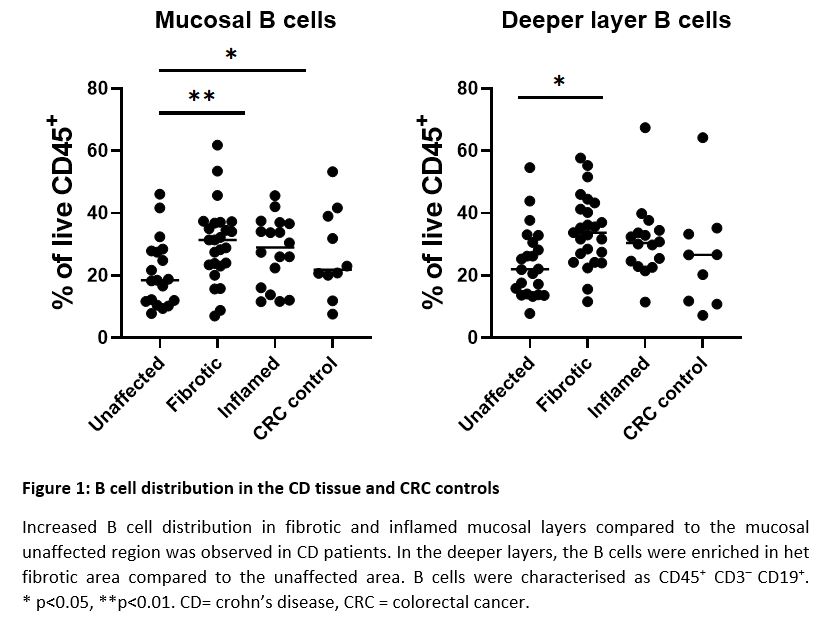
Flow cytometric analysis in CD patients showed mainly B cell enrichment in fibrotic tissue compared to the unaffected tissue, both in the mucosa (30.2% vs 20.9% of CD45+ cells, p=0.001) as in deeper layers (34.4% vs 24.1% of CD45+ cells, p=0.01) (figure 1). Mature dendritic cells (mDC), alternatively-activated macrophages and eosinophils were also enriched but in the deeper layers of the fibrotic segment compared to the mucosa (4.3% vs 3.4%, 1.07% vs 0.04% and 2.3% vs 1.5% of CD45+ cells; p = 0.02, 0.008 and 0.02) (figure 2). Moreover, increased eosinophilic presence was also shown in the unaffected CD region compared to the fibrotic area, both in deeper layers (4.0% vs 2.3% of CD45+ cells; p=0.03) as the mucosa (2.4% vs 1.5% of CD45+ cells; p=0.01) as well as in the inflamed deeper layers compared to the superficial layers (3.3% vs 2.1% of CD45+ cells, p=0.04). These data were corroborated by increased CCL11 mRNA expression, in the deeper layers of fibrotic tissue compared to the mucosa (52.3% vs 31.0%, p=0.03).
Conclusion
Our results suggest a role for innate immune cells in CD complicated with fibrosis. Especially in the deeper layers, eosinophils, mature dendritic cells and alternatively-activated macrophages are implicated. The eosinophilic enrichment in the deeper layers may partly be attributed to an increased eosinophil recruitment by CCL11. Our results offer opportunities for anti-fibrotic drug development in CD.