P095 Newly discovered gut matrikines from Crohn’s disease intestinal tissue induce cell proliferation, activation and inflammatory response in intestinal myofibroblasts in vitro
Papatheodoridi, M.(1)*;Giuffrida , P.(1);Canetti, D.(2);Yutaka, Y.(3);Hodgetts, H.(1);Di Sabatino, A.(4);Johnston, H.(5);Verona, G.(6);Rendell, N.(6);Al-Akkad, W.(1);Frenguelli, L.(1);Hall, A.(1);Luong , T.(1);Sampietro, G.(7);Pinzani, M.(1);Rombouts, K.(1);Belloti, V.(6);Mazza, G.(1);
(1)University College London, Institute for Liver and Digestive Health, London, United Kingdom;(2)University College London, Centre for Amyloidosis and Acute Phase Protein, London, United Kingdom;(3)Institute for Liver and Digestive Health, University College London, London, United Kingdom;(4)University of Pavia- San Matteo Hospital Foundation, First Department of Internal Medicine, Pavia, Italy;(5)UCL EGA Institute for Women's Health, Cancer Proteomics- Women's cancer-, London, United Kingdom;(6)University College London, Centre for Amyloidosis and Acute Phase Proteins, London, United Kingdom;(7)Università degli Studi di Milano, ivision of General and HPB Surgery, Milan, Italy;
Background
The interaction between intestinal myofibroblasts (iMFBs) and extracellular matrix (ECM) changes contributes to Crohn’s disease (CD) fibrosis, but the exact mechanisms are not yet clear. Our group recently discovered a selection of matrix-derived peptides (matrikines) that specifically appear in the intestinal tissue of patients with CD fibrostenosis.1 Here, we evaluate the in vitro effect of 19 matrikines (with various bioactivity likelihood) on primary human intestinal myofibroblasts (IMFBs), key cells in CD fibrosis.
Methods
Primary human iMFBS (500K/mL, Passage 4, Novabiosis) were cultured in serum-free medium (SFM) (DMEM high glucose, 1% sodium pyruvate, 1% non-essential aminoacids, 1% Antibiotics-Antimycotics and 3mg Gentamicin), complete medium (SFM with 10% fetal bovine serum), PDGF-bb 10ng/mL or treated with each of the matrikines at 10, 50, 100 μg/mL for 24hours. The effect of each matrikine on cell proliferation, gene expression and migration was assessed by BrdU, qPCR and Transwell assays, respectively. RNA sequencing data was analysed using the GSEA software.
Results
The effect on iMFBs proliferation and viability was tested with each of the 19 matrikines at 3 different doses (Table 1). 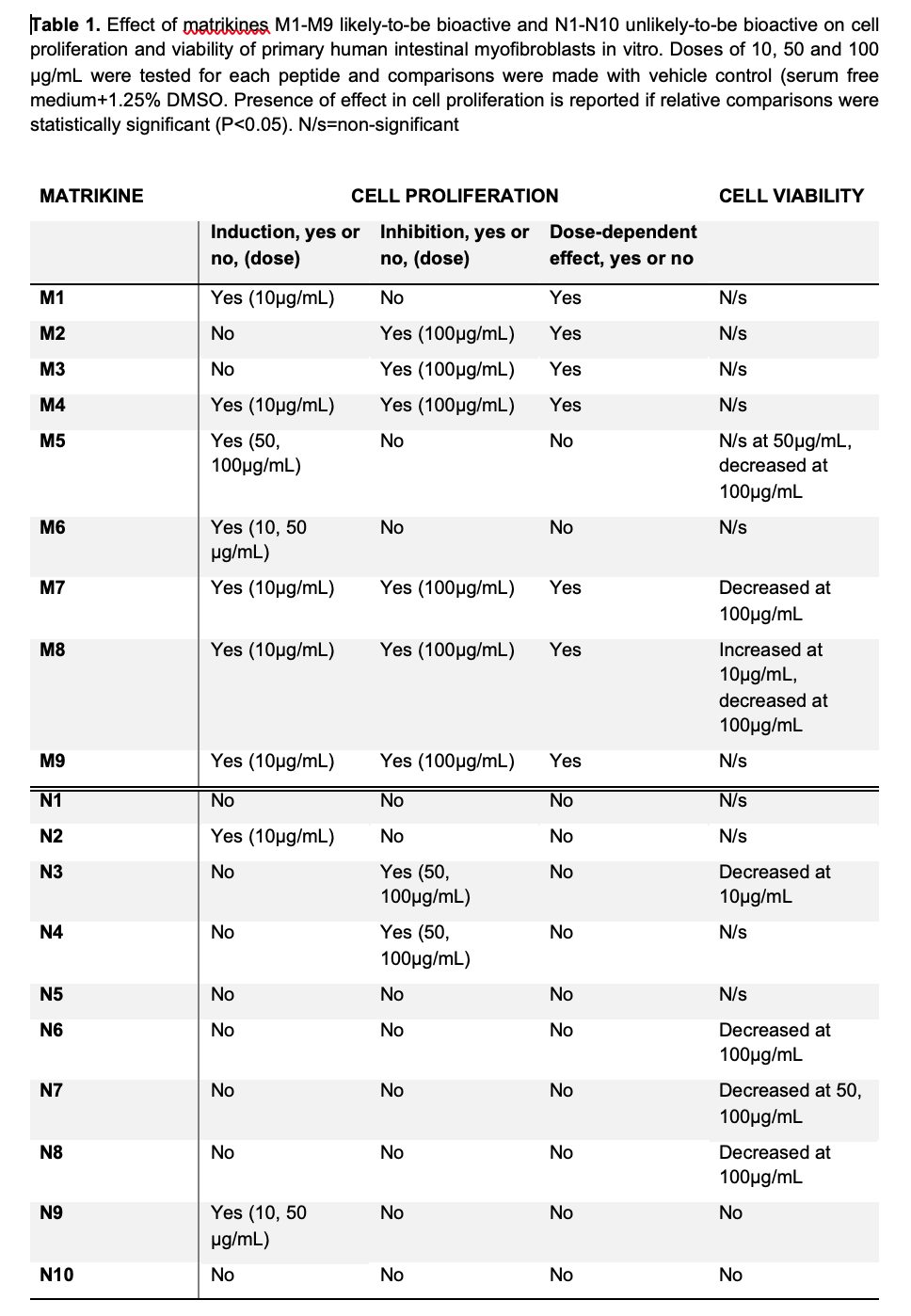
Among 9 most likely-bioactive matrikines (M1-M9) (Figure 1), M1, M4, M7, M8 and M9 induced the highest effect in cell proliferation and were selected for further experiments. Gene expression assays showed upregulation of ACTA2 with M1 or M7 and upregulation of COL1A1 with M1, M7 or M8, indicating activation of the iMFBs (Figure 2). 
 Transwell assays also showed that M1, M4 and M8 induce iMFB cell migration.
Transwell assays also showed that M1, M4 and M8 induce iMFB cell migration.
A total of 1948, 1024, 460, 570, and 2749 differentially expressed genes (DEGs) were identified in iMBFs treated with M1, M4, M7, M8 and M9 vs no treatment, showing enrichment in pathways related to “smooth muscle contraction”, “fibroblast growth factor”, “cell migration”, “collagen formation”, “elastin formation” and “ECM organization”; most of which were also enriched by complete medium and/or PDGFbb that served as positive controls. However, pathways related to “proinflammatory and profibrotic mediators” or “vascular permeability” were only enriched in iMFBs treated with M1, M4, M8 and M9 or M1 and M9, respectively (Figure 3).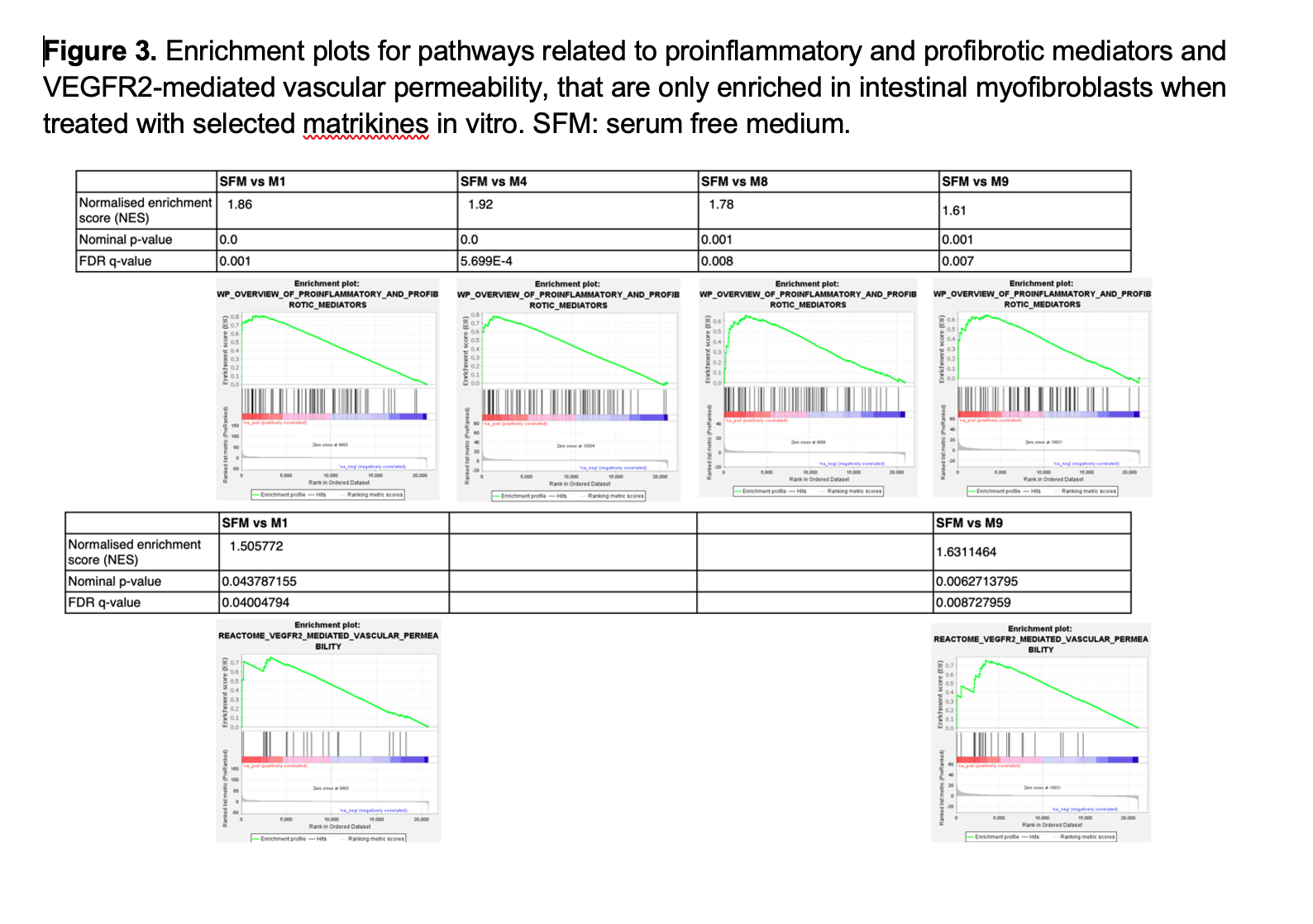
Conclusion
A selection of newly discovered CD-specific matrikines induce activation of iMFBs as well as inflammatory response and endothelial changes, suggesting an important role and therefore their potential value as biomarkers in the progression of intestinal fibrostenosis.


