P100 Atypical Anti-Neutrophil Cytoplasmatic Antibodies Are Antibodies Against Neutrophil Extracellular Traps: A Novel Pathophysiological Mechanism In Ulcerative Colitis
Mendieta Escalante, E.A.(1)*;Faber, K.N.(1);Hermoso Ramello, M.(1);Parada-Venegas, D.(1);Bourgonje, A.(2);Roozendaal, C.(3);Dijkstra, G.(1);
(1)University Medical Center of Groningen, Gastroenterology and Hepatology, Groningen, The Netherlands;(2)University Medical Center Groningen, Gastroenterology and Hepatology, Groningen, The Netherlands;(3)University Medical Center Groningen, Department of Laboratory Medicine, Groningen, The Netherlands;
Background
The number of mucosal neutrophils, as well as the presence of neutrophil extracellular traps (NETs), correlate with disease activity in Ulcerative colitis (UC), an inflammatory bowel disease. Most (~80%) UC patients present atypical Anti-Neutrophil Cytoplasmatic Antibodies (a-ANCAs) to a yet unknown antigen, which shows a different immunofluorescence staining pattern when compared to cytoplasmatic (c-ANCA) and perinuclear (p-ANCA) that target proteinase (PR3) and myeloperoxidase (MPO), respectively. Here, we analyzed whether a-ANCAs specifically bind to NETs.
Methods
Blood neutrophils were treated to form non-lytic NETs using LPS-treated platelets with and without DNAse and Trypsin. Patient biopsies and in vitro-formed non-lytic NETs were incubated with p-ANCA, c-ANCA and a-ANCA positive patient and ANCA negative control serum and processed for immunofluorescence microscopy. Macrophage-mediated clearance of serum-pretreated NETs was analyzed in real-time using Incucyte microscopy.
Results
Ethanol-fixed neutrophils displayed c-ANCA, p-ANCA patterns, and a-ANCA web-like staining. Only c- and p-ANCA react to DNAse-treated ethanol-fixed neutrophils (Fig 1). Serum containing a-ANCA’s bound to non-lytic NETs that disappeared upon treatment with DNAse and Trypsin whereas c- and p- ANCA binding did not disappear upon DNAse treatment (Fig 2) indicating that both DNA and proteins are needed for a-ANCAs to bind to non-lytic NETs. In inflamed colonic tissue of UC patients, a-ANCA co-stained with extracellular DNA and neutrophil elastase covering the intestinal epithelium (Fig 3). In vitro, NETs were efficiently cleared by macrophages, which was strongly inhibited after pre-incubating with a-ANCAs (Fig 4). Macrophages exposed to a-ANCA-incubated NETs expressed higher CXCL-8 and IFN-B levels than the NETs exposed to control serum (both p<0.05).
Figure 1
Figure 2
Figure 3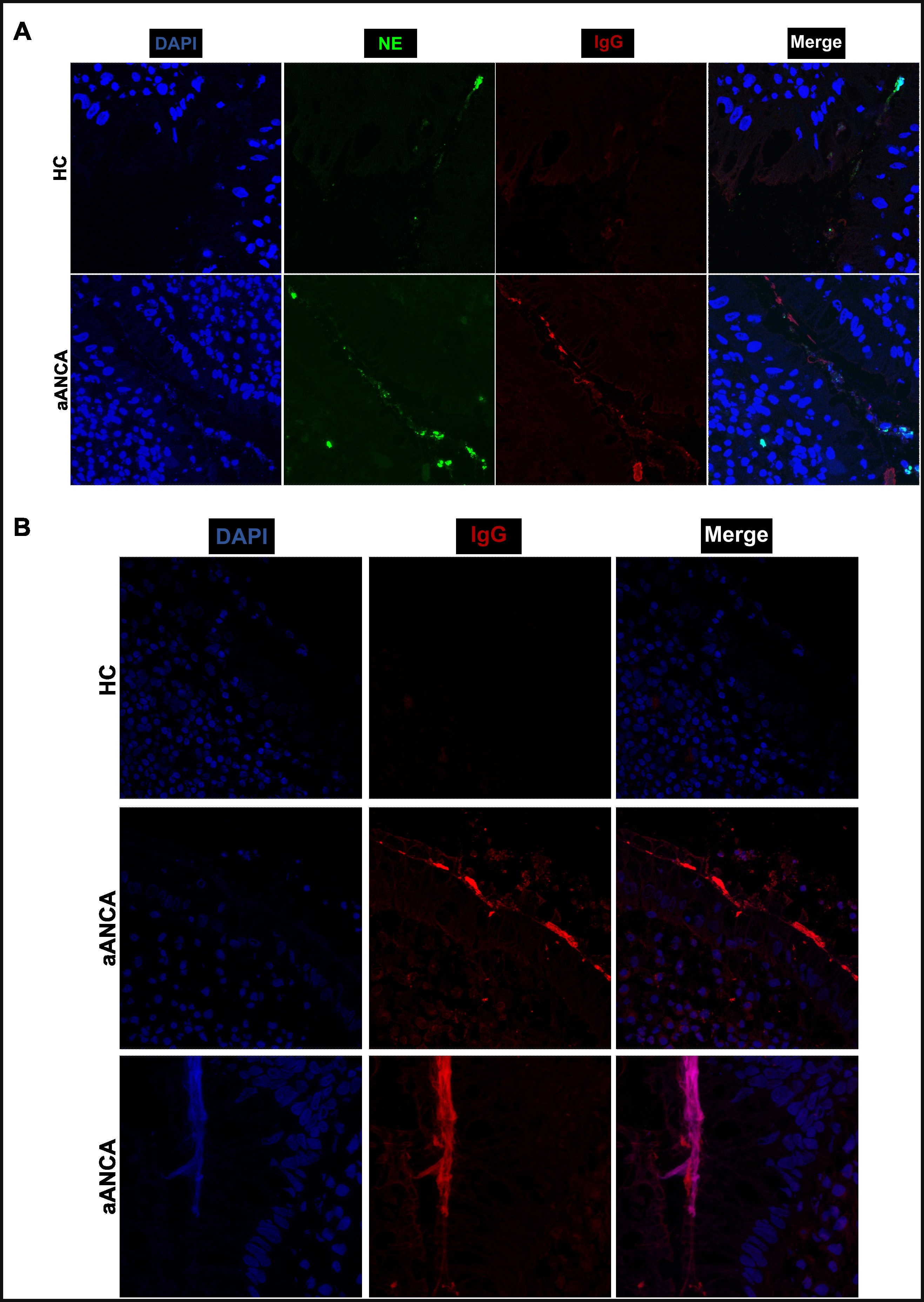
Figure 4
Conclusion
a-ANCAs specifically bind to de novo DNA-protein antigens in non-lytic NETs, which prevents efficient macrophage-mediated clearance and induces a pro-inflammatory (M1) phenotype. This inflammatory milieu may contribute to the pathophysiology of UC.


