P102 A small molecule selective integrin α4β7 inhibitor demonstrates efficacy in a chronic model of Inflammatory Bowel Disease
Redhu, N.S.(1)*;Rowe, M.(1);Lee, D.(2);Jain, D.(1);Sang, A.(1);Granata, D.(1);Harrison, B.(3);Cui, D.(2);Bursavich, M.(3);Ray, A.S.(1);Lippa, B.(3);Rogers, B.N.(3);Wong , J.(1);
(1)Morphic Therapeutic, Biology and Translation, Waltham, United States;(2)Morphic Therapeutic, Drug Metabolism and Pharmacokinetics, Waltham, United States;(3)Morphic Therapeutic, Chemistry, Waltham, United States;
Background
Integrin α4β7 regulates the recruitment of T cells to intestinal mucosa through its interaction with mucosal addressin cell adhesion molecule (MAdCAM)-1. Disruption of this interaction has been clinically validated for the treatment of inflammatory bowel diseases (IBD) by the anti-α4β7 antibody vedolizumab. The current study was aimed at elucidating the preclinical efficacy of MT-103, a potent and selective small molecule α4β7 inhibitor in the clinically relevant CD4+CD45RBhi T cell transfer (TCT) colitis mouse model.
Methods
Mice were administered either MT-103 via subcutaneously implanted minipumps across 3 dose cohorts (3, 10, or 30 mg/ml), or an antibody specific for IL12p40 (25 mg/kg) or α4β7 (30 mg/kg), through intraperitoneal injections for 7 weeks. Colitis development was evaluated via readouts including body weights, gross colon weight by length ratios, colonic tissue gene expression, and histopathological scores.
Results
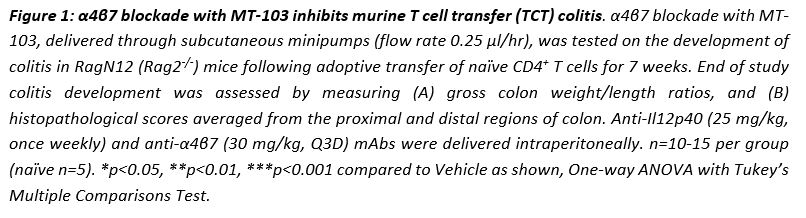
MT-103 significantly inhibited the development of colitis in mice at all 3 doses equivalently, as evident from significantly improved gross colon weight/length ratios in comparison to vehicle controls which presented with moderate-severe colitis (Figure 1A). MT-103 treatment also led to a significant protection from weight loss that occurred in vehicle-treated mice. Importantly, mice treated with MT-103 exhibited remarkably improved histopathology scores, including inflammation, hyperplasia, and gland loss compared to vehicle controls (Figure 1B). Furthermore, broad gene transcriptional analysis showed that MT-103 broadly suppressed pro-inflammatory pathways and processes within colonic tissues. Specifically, MT-103-induced downregulation of Ifng, Il17a, and Il1b, besides reduced Cd3 as a surrogate metric of T cell trafficking inhibition to the colon, and conversely enabled upregulation of anti-inflammatory cytokine Il10. The colitis-protective effects of MT-103 were equivalent, if not superior, to the saturating doses of α4β7 mAb or anti-IL12p40 mAb when compared to their respective vehicle controls.
Conclusion
Treatment with MT-103 protects from colitis by restoring colonic tissue homeostasis as measured by body fitness and an array of immunological and histopathological assessments. These proof-of-concept data demonstrate an α4β7-specific small molecule, MT-103, can occlude pathogenic T cells from initiating disease in a chronic IBD model, equivalent to an α4β7 blocking antibody.