P103 Colitis-associated faecal miRNAs are mediators of host-microbiota interactions and key regulators of intestinal permeability and inflammation
Casado-Bedmar, M.(1)*;Roy, M.(1);Hugot, J.P.(1);Le Gall, M.(1);Chassaing, B.(2);Viennois, E.(1);
(1)INSERM, Center of Research on Inflammation CRI, Paris, France;(2)INSERM, Mucosal microbiota in chronic inflammatory diseases CNRS UMR 8104, Paris, France;
Background
Like in other chronic pathologies, evidence has shown disruption of host immune-microbial interactions and intestinal dysbiosis in the pathogenesis of Inflammatory Bowel Diseases (IBD), also characterized by chronic inflammation and increased intestinal permeability. Faecal microRNAs (miRNAs) have recently emerged as potential inter-kingdom mediators between the host and its microbiota, but the influence of its interaction on chronic intestinal disorders remains unclear. Here, we aimed to study the impact of colitis-associated miRNAs on intestinal inflammation, permeability and microbiota.
Methods
A panel of faecal miRNAs was profiled in IL10-/- mice, known as a model of spontaneous colitis. Faecal miRNAs and microbiota composition were compared between IBD and healthy controls. Identified colitis-associated miRNAs were then administered with/out antibiotics ad libitum to C57BL6 mice. Parameters of inflammation, intestinal epithelial barrier function, as well as microbiota composition, were analysed. Ex vivo and in silico studies were performed to comprehend the direct effects of the miRNAs on the gut microbiota and the host. Lastly, endogenous miRNAs were inhibited in IL10-/- mice administering miRNA-inhibitors through oral gavage.
Results
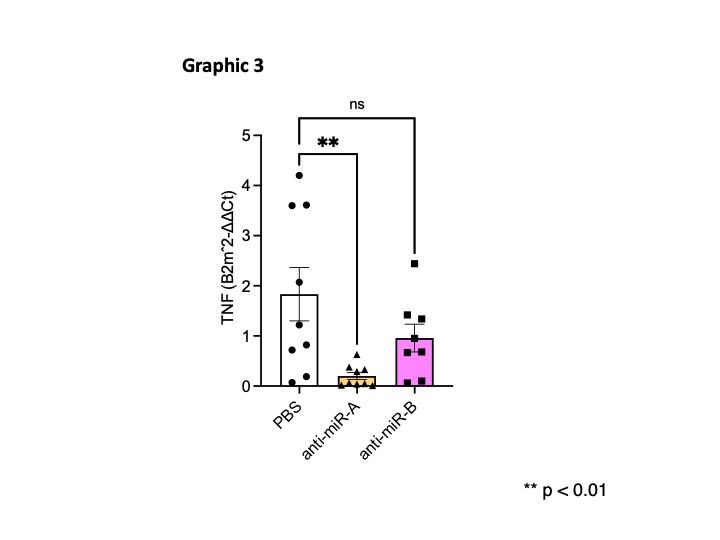
We identified in mice two colitis-associated miRNAs that were also increased in faecal samples of IBD compared to healthy controls (Graphic 1). Notably, the exogenous administration of these two miRNAs was sufficient to drive intestinal inflammation (Graphic 2) and alter the intestinal barrier function in mice. Such effects were directly impacted by the microbiota presence and composition. In humans, the measurement of faecal lipopolysaccharides and the 16S microbiota sequencing further showed strong associations between miRNAs and bacterial species, such as Faecalibacterium prausnitzii and Parabacteroides distasonis. In mice, the treatment with colitis-associated miRNAs altered the microbiota composition with a significant increase of Cyanobacteria and Verrucomicrobiota. Importantly, the inhibition of endogenous miRNAs through anti-miRNA administration prevented the emergence of severe colitis in IL10-/- mice (Graphic 3).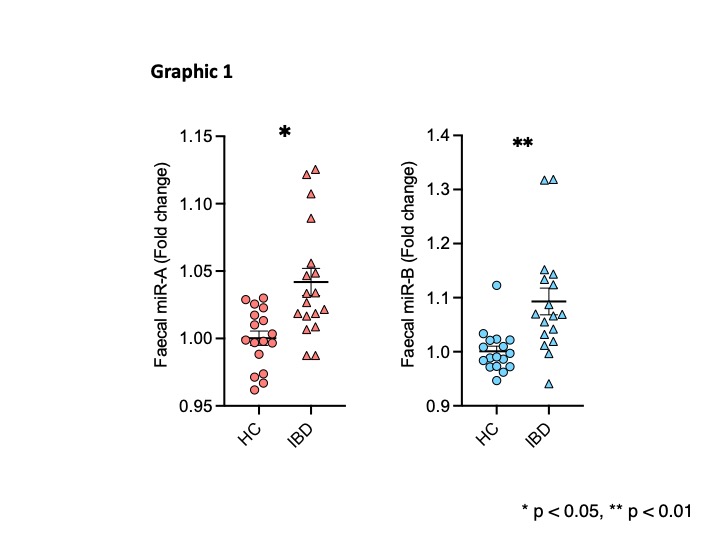

Conclusion
Overall, our findings identify an inflammatory role played by faecal miRNAs in the gastrointestinal environment, which pathogenic potential appears closely related to dysbiosis. Hence, our data suggest that miRNAs or miRNA inhibitors might be considered therapeutic agents to beneficially alter the microbiota and intestinal inflammation in IBD and other dysbiosis-associated conditions.


