P113 Protein fingerprint biomarkers of collagen remodeling can evaluate fibrogenesis in an in vitro colonic scar-in-a-jar model
Pehrsson, M.(1)*;Sorokina Alexdottir, M.(1);Asser Karsdal, M.(2);Høg Mortensen, J.(1);
(1)Nordic Bioscience A/S, Gastroenterology, Herlev, Denmark;(2)Nordic Bioscience A/S, Biomarkers and Research, Herlev, Denmark;
Background
Intestinal fibrosis affects most inflammatory bowel disease (IBD) patients, resulting in severe clinical complications and reduced treatment response. With no treatments approved for intestinal fibrosis, there is a need for preclinical models to investigate the pathobiology and novel treatments. Myofibroblasts are the main drivers of intestinal fibrosis and the excessive accumulation of extracellular matrix in IBD patients. We investigated a prolonged scar-in-a-jar model of colonic fibrogenesis to validate protein fingerprint (PF) biomarkers of collagen formation for clinical use and the in vitro model as a screening tool.
Methods
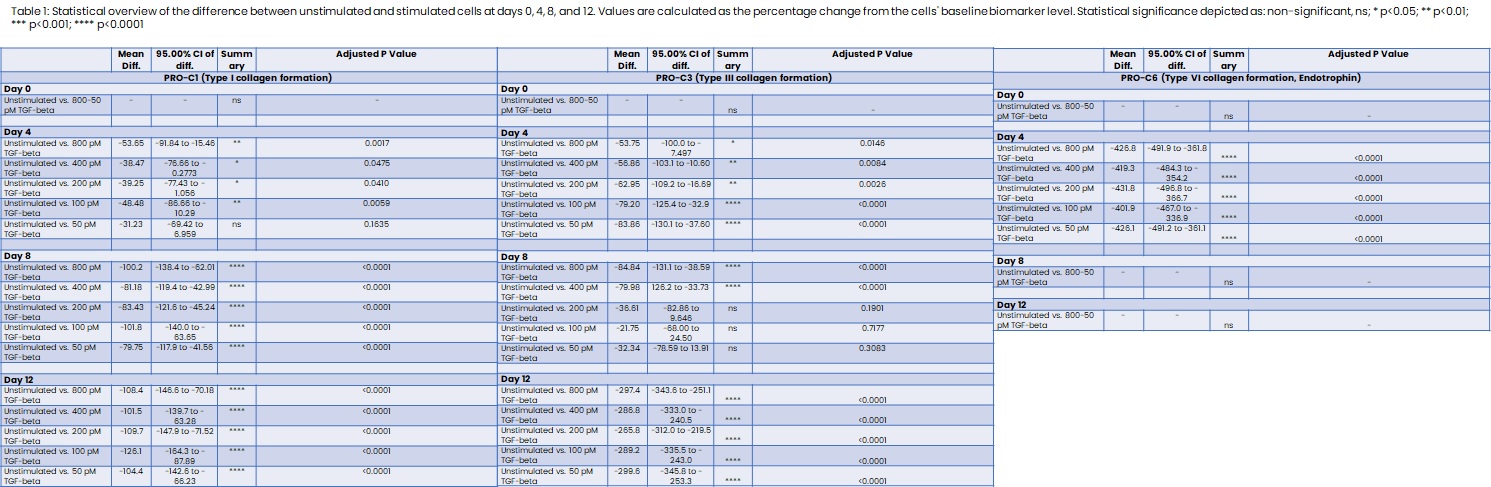
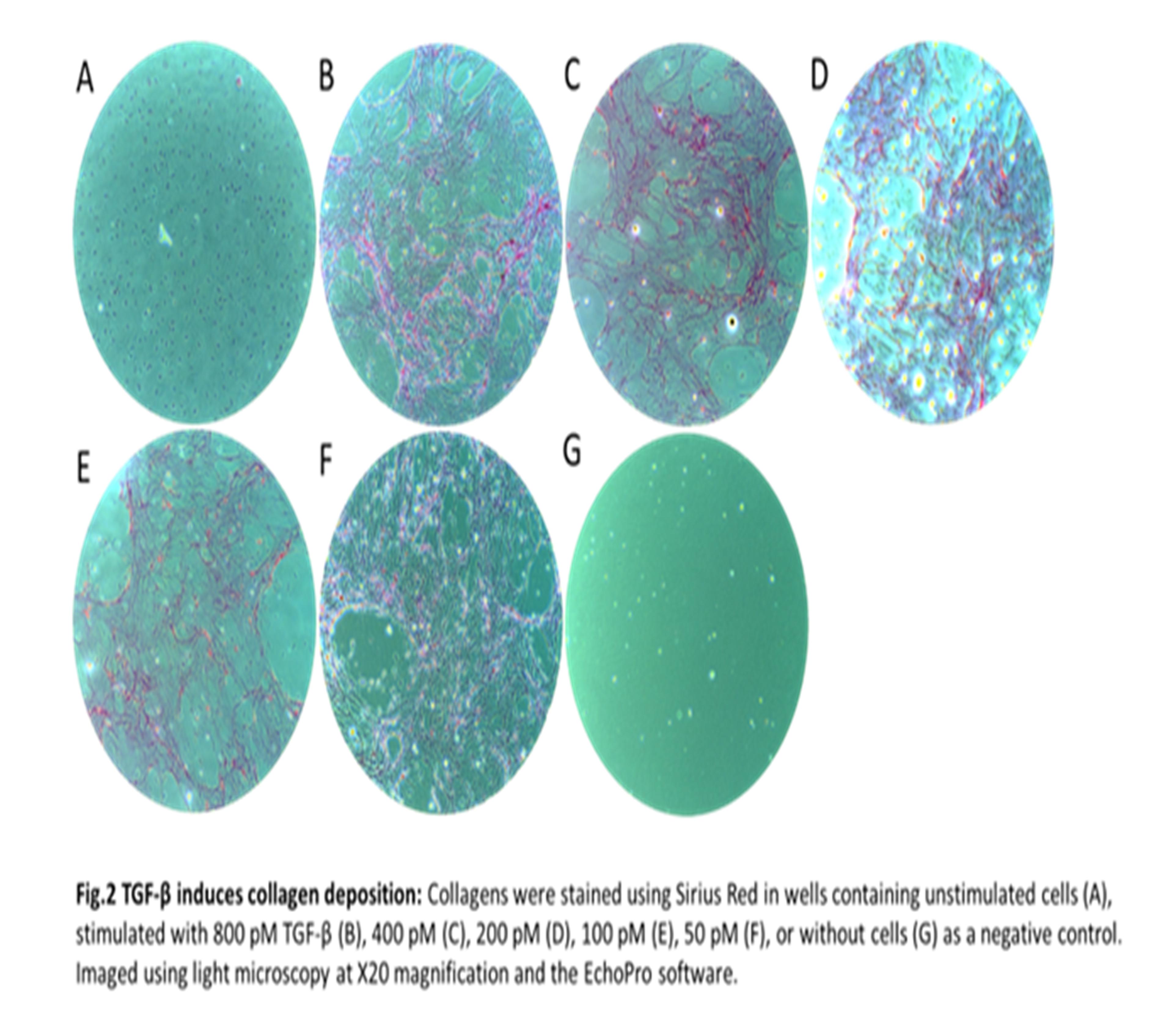
Human colonic fibroblasts (CCD-18co) purchased from ATCC were grown in EMEM supplemented with 10% FBS and 1% P/S in culture flasks with 200 µg/cm3 gelatin to reduce cell activation. At 80% confluency, the cells were seeded at 30,000 cells/well in a 48-well plate noted as day -2. The cells were either unstimulated or stimulated with a 2-fold dilution of TGF-β starting at 800 pM to 50 pM starting at day 0 and then every fourth day, terminating at day 12. At termination, the wells were decellularized and the collagen matrix was stained with Sirius Red for visual confirmation of collagen formation. Imaging was done using light microscopy at X20 magnification using similar settings for all images. Fibrogenesis was quantitatively determined by measuring the biomarkers PRO-C1, PRO-C3, and PRO-C6 in the supernatants. The biomarkers reflect type I, type III, and type VI collagen formation, respectively. We applied Šídák's multiple comparisons test for differences in the collagen formation biomarker levels by comparing stimulated with unstimulated cells.
Results
Stimulation with TGF-β significantly increased type I and type III collagen formation at days 4, 8, and 12 compared to unstimulated cells, with the stimulation inducing a 297.4% increase of type III collagen at day 12 (table 1 + fig.1). Irrespective of the TGF-β concentration, a 426.8% spike in the fibrotic hormone endotrophin (PRO-C6) was observed on day 4, which declined to unstimulated levels at days 8 and 12 (table 1 + fig.1). All concentrations of TGF-β induced a significant release of the collagen formation biomarkers to the supernatant (fig. 1). Collagen deposition was confirmed by Sirius Red (fig. 2). No significant increase was observed in the unstimulated cells determined by biomarker measurements and Sirius Red (fig. 1+2).

Conclusion
PF biomarkers can be applied in vitro to objectively quantify fibrogenesis, demonstrating a significant increase following TGF-β stimulation of colonic fibroblasts. The model may be combined with the biomarkers to evaluate drug targets of intestinal fibrosis.


