P150 Identification of features associated with poor outcomes in pediatric patients with ulcerative proctitis: A Multicentre Study From the Paediatric IBD Porto Group of ESPGHAN
Tal-Shifman, N.(1,2)*;Tzivinikos, C.(3);Gasparetto, M.(4);Serban, D.E.(5); Zifman, E.(6); Hojsak, I.(7); Ledder, O.(8); Yerushalmy Feler, A.(9); Rolandsdotter, H.(10); Aloi, M.(11);Bramuzzo, M.(12); Buderus, S.(13);Lionetti, P.(14);Norsa, L.(15); Norden, C.(16);Urlep, D.(17); Romano, C.(18);Shaoul, R.(19);Martinez-Vinson, C.(20);Karoliny, A.(21);Veereman, G.(22); Kang, B.(23);Vlčková, E.(24); Alvisi, P.(25);Kori, M.(26);Tavares, M.(27);Weiss, B.(28);Hussey, S.(29);Essen Qamhawi, M.(30);Palomino Pérez, L.M.(31);Henderson, P.(32); Parmar, R.(33); Miele, E.(34);Firas Rinawi, F.(35);Lonzano-Ruf, A.(36); Zamvar, V.(37); Kolho, K.L.(38);Shouval, D.(1,2);
(1)Schneider Children’s Medical Center of Israel, Institute of Gastroenterology- Nutrition and Liver Diseases, Petah Tikva, Israel;(2)Tel Aviv University, Sackler Faculty of Medicine, Tel Aviv, Israel;(3)Al Jalila Children's Specialty Hospital- Mohammed Bin Rashid University- Dubai Medical College, Pediatric Gastroenterology Department, Dubai, United Arab Emirates;(4)Barts Health NHS Trust- The Royal London Children's Hospital, Department of Paediatric Gastroenterology, London, United Kingdom;(5)“Iuliu Hatieganu” University of Medicine and Pharmacy- Emergency Clinical Hospital for Children, 2nd Clinic of Pediatrics, Cluj-Napoca, Romania;(6)Meir medical center- Kfar-Saba- Israel, Pediatric Gastroenterology Unit, Kfar-Saba, Israel;(7)Children's Hospital Zagreb- Zagreb, Referral Center for Pediatric Gastroenterology and Nutrition, Zagreb, Croatia;(8)Shaare Zedek Medical Center- The Hebrew University of Jerusalem, The Juliet Keidan Institute of Pediatric Gastroenterology & Nutrition, Jerusalem, Israel;(9)"Dana-Dwek" Children's Hospital- Tel Aviv Sourasky Medical Center, Pediatric Gastroenterology Institute, Tel aviv, Israel;(10)Sachs' Children and Youth Hospital Södersjukhuset, Department of Gastroenterology, Stockholm, Sweden;(11)Sapienza University of Rome, Pediatric Gastroenterology- Hepatology and Nutrition Institute, Rome, Italy;(12)IRCCS “Burlo Garofolo”, Institute for Maternal and Child Health, Trieste, Italy;(13)St-Marien-Hospital, Department of Pediatrics, Bonn, Germany;(14)University of Florence- Meyer Children's Hospital, Department NEUROFARBA, Florence, Italy;(15)ASST Papa Giovanni XXIII, Pediatric Hepatology- Gastroenterology and Transplantation Unit, Bergamo, Italy;(16)Hvidovre University Hospital, Department of Pediatrics, Copenhagen- Hvidovre, Denmark;(17)University Children's Hospital of the University Medical Centre Ljubljana, Pediatric Gastroenterology and Liver Unit, Ljubljana, Slovenia;(18)G. Barresi-' University of Messina, 8Pediatric Gastroenterology and Cystic Fibrosis Unit- Department of Human Pathology in Adulthood and Childhood, Messina, Italy;(19)Ruth Children's Hospital of Haifa- Rambam Medical Center, Pediatric Gastroenterology and Nutrition Institute, Haifa, Israel;(20)Hopital Robert Debré- Assistance Publique Hopitaux de Paris., Department of Pediatric Gastroenterology and Nutrition, Paris, France;(21)Heim Pál National Pediatric Institute-, Institute of Gastroenterology- Nutrition and Liver Disease, Budapest, Hungary;(22)Kidz Health Castle UZ Brussels- Free University Brussels- Belgium, Department of Paediatric Gastroenterology and Nutrition, Brussels, Belgium;(23)School of Medicine- Kyungpook National University, Department of Pediatrics, Daegu, Korea- Republic Of;(24)2nd Medical Faculty- Charles University and University Hospital Moto, Department of Pediatrics, Prague, Czech Republic;(25)Maggiore Hospital, Pediatric Gastroenterology Unit, Bologna-, Italy;(26)Kaplan Medical Centre- Rehovot and the Faculty of Medicine- Hebrew University of Jerusalem, Pediatric Gastroenterology, Jerusalem, Israel;(27)Centro Materno Infantil do Norte- Centro Hospitalar e Universitário do Porto, Department of Pediatric Gastroenterology, porto, Portugal;(28)Edmond and Lily Safra Children's Hospital, Division of Pediatric Gastroenterology and Nutrition-, Tel Hashomer, Israel;(29)Children's Health Ireland- UCD and RCSI, Department of Gastroenterology- Nutrition and Liver Disease, Dublin, Ireland;(30)Astrid Lindgren Children’s Hospital- Karolinska University Hospital, Department of Gastroenterology- Hepatology and Nutrition, Stockholm, Sweden;(31)Hospital Infantil Universitario Niño Jesús, Gastroenterology and Nutrition Department, Madrid, Spain;(32)Royal Hospital for Children and Young People, Department of Paediatric Gastroenterology and Nutrition, Edinburgh, United Kingdom;(33)great North Children's Hospital, Department of Pediatric Gastroenterology, Newcastle, United Kingdom;(34)University of Naples "Federico II"-, Department of Translational Medical Science- Section of Pediatrics, Naples, Italy;(35)Emek Medical Center, Pediatric Gastroenterology Unit, Afula, Israel;(36)Hospital Sant Joan de Déu, Department of Pediatric Gastroenterology- Hepatology and Nutrition, barcelona, Spain;(37)Leeds Children's Hospital- Leeds Teaching Hospitals NHS Trust, Department of Paediatric Gastroenterology, Leeds, United Kingdom;(38)Tampere University Hospital and University of Tampere- Finland and Children's Hospital- University of Helsinki, Department of Paediatric Gastroenterology, Helsinki, Finland;
Background
Ulcerative proctitis (UP) is an uncommon presentation in paediatric patients with ulcerative colitis, accounting for <10% of cases. Here we aimed to characterize the clinical features and natural history of paediatric patients with UP, and to identify predictors of poor outcomes.
Methods
A retrospective cohort study involving 37 sites affiliated with the IBD Interest group of ESPGHAN. Data were collected at different timepoints from patients diagnosed with UP aged <18 years between 01/01/2016-31/12/2020. Outcomes included time to initiation of systemic steroids, thiopurines or biologics, time to acute severe colitis (ASC), IBD-related admission and colectomy. Univariate cox regression was used to study the association between potential predictors and study outcomes.
Results
Two hundred and fifty patients with UP were included, with a median age at diagnosis of 14.5 (IQR 12.3-15.9) years, and a median follow-up of 2.7 (IQR 1.7-3.9) years. The most common presenting symptoms were bloody stools (94%), abdominal pain (59%) and diarrhea (53%). At diagnosis, the median pediatric ulcerative colitis activity index (PUCAI) score was 25 (IQR 20-35), and only 3 patients (1.3%) presented with ASC. Most children had normal inflammatory markers and albumin levels. the median fecal calprotectin level was 720 mcg/g (IQR 310-1800), while 16 patients (9.9%) had a calprotectin level <100mcg/g at diagnosis. Administration of oral or topical 5-ASA resulted in clinical remission rates of 52% and 48%, respectively, by the end of induction, while the combination of both led to remission in 73% of patients. The rates of treatment escalation to thiopurines and biologics at 1, 3 and 5 years were 11%, 27% and 45%, and 11%, 23% and 45%, respectively (Figure 1). Within 5 years from diagnosis, 20% of patients had presented with ASC. The PUCAI score at diagnosis of UP was highly associated with initiation of systemic steroids, thiopurines or biologics, as well as later ASC event and IBD admission, while a Mayo endoscopic score of 3 was associated with the initiation of biologics and subsequent ASC event and IBD admission (Table 1). Seven patients (4%) underwent colectomy by the end of follow-up. Among the 151/250 patients who had a repeat endoscopy, only 16% achieved mucosal healing and in 48% inflammation extended proximally. Cecal patch (P=0.009) and higher PUCAI score (P=0.009) at diagnosis, as well as higher PUCAI score (P=0.009) and lack of steroid-free clinical remission (P=0.005) by the end of induction were associated with proximal disease extension.
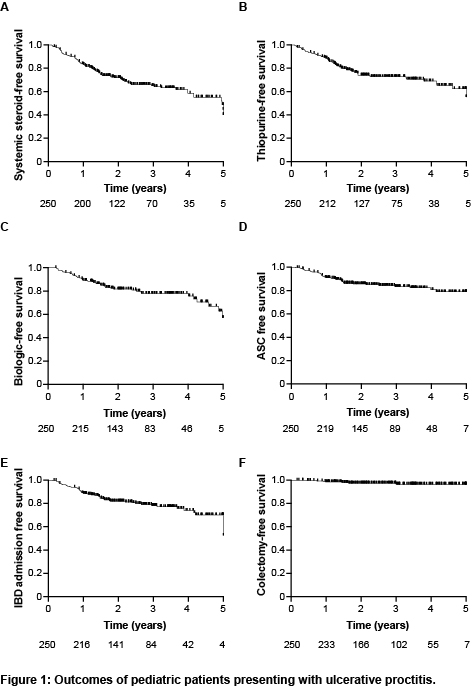
Conclusion
Disease burden is significant in paediatric patients that present with UP, with high rates of proximal disease extension and requirement for treatment escalation.


