P170 Point-of-care ultrasound accurately quantifies skeletal muscle index in patients with Inflammatory Bowel Disease
Nguyen, A.(1);Burns, M.(2);Lambell, K.(3);Holt, D.(2);Herath, M.(4);Gibson, P.R.(5);Ebeling, P.(6);Milat, F.(4);Moore, G.(7);
(1)Monash University- Monash Health and Alfred Health, Gastroenterology, Melbourne, Australia;(2)Monash Health, Gastroenterology, Melbourne, Australia;(3)Alfred Health, Nutrition, Melbourne, Australia;(4)Monash University- Monash Health and Hudson Institute of Medical Research, Endocrinology, Melbourne, Australia;(5)Monash University and Alfred Health, Gastroenterology, Melbourne, Australia;(6)Monash University and Monash Health, Endocrinology, Melbourne, Australia;(7)Monash University and Monash Health, Gastroenterology, Melbourne, Australia;
Background
Body composition is frequently altered in patients with Inflammatory Bowel Disease (IBD) and difficult to quantify without expensive and radiation-prone techniques. Low skeletal muscle index (SMI) has been associated with increased surgical complications and poor quality of life. Aim: To develop a portable ultrasound (US) protocol to estimate SMI, to compare this with SMI from paired whole-body dual-energy X-ray absorptiometry (DXA) in patients with IBD and controls, and to assess its responsive to change over time.
Methods
Adult patients with IBD and gender-matched controls underwent prospective assessment of body composition by US (Philips Lumify) and DXA (GE Lunar Prodigy) for which low SMI was defined as men ≤7.26 kg/m2 and women ≤5.45 kg/m2. Sonographic muscle thickness of the dominant arm and bilateral thighs (Figure 1) were multiplied by respective bone lengths (humerus or femur) and described in cm2.
Results
Results were analysed by Spearman correlation and receiver operating characteristic (ROC) curves using just arm measurements (USarm) or combined arm and thigh measurements (UScombined). A subgroup of patients with active IBD (faecal calprotectin of ≥150 µg/g) were assessed again ≥14 weeks after initiating biologic induction therapy. Response was defined with faecal calprotectin reduction of ≥50%.
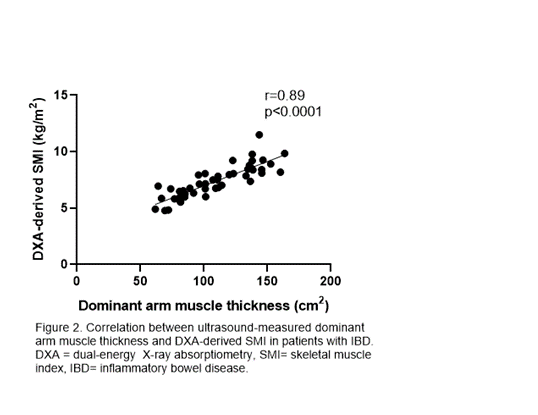
48 patients with IBD had similar SMI compared with 30 controls (7.1 vs 7.7 kg/m2), but a higher proportion had low SMI (19% vs. 3%, p=0.048). In both IBD and control participants, DXA-derived SMI was strongly associated with USarm measurements (r=0.89 and r=0.88, p<0.0001) and UScombined measurements (r=0.90 and r=0.87, p<0.0001).
On ROC analysis in patients with IBD, low SMI was identified best with USarm <73 cm2 in women (AUC 0.93, sensitivity 100%, specificity 89%) and <117 cm2 in men (AUC 0.88, sensitivity 100%, specificity 81%). In 7 of 14 patients who responded to biologic induction, only DXA-SMI increased significantly (7.9 to 9 kg/m2) compared to USarm (109 to 124 cm2, p=0.249) and UScombined (270 to 323 cm2, p=0.080). Including both responders and non-responders, change in UScombined correlated well with change in DXA-SMI (r=0.77, p=0.007).

Conclusion
Sonographic measurement of the dominant arm ± bilateral thigh muscle thickness accurately assesses DXA-derived SMI at the point-of-care. In preliminary evaluation, it was responsive to change after biologic induction therapy, but larger sample sizes and longer follow up are needed to evaluate its monitoring role.


