P179 Treatment decisions and biologic adoption rates in newly diagnosed Crohn's disease in Japan
Hisamatsu, T.(1);Yoon, A.(2);
(1)Kyorin University School of Medicine, Department of Gastroenterology and Hepatology, Tokyo, Japan;(2)Takeda Pharmaceutical Company, Japan Medical Office, Tokyo, Japan
Background
Biologic therapy in Crohn’s disease (CD) has significantly improved treatment outcomes. Still, providing an optimal care for individual patient in real-world is complex. We report the real-world treatment decisions between biologics and systemic corticosteroids (SCS) and associated factors in newly diagnosed CD in Japan.
Methods
Patients with CD diagnosis (Index) and at least one CD therapeutic agent prescribed between 2006 and 2019 were screened from the JMDC database. JMDC is a health claims database where various sized (small to very large) companies’ employees and their dependents are enrolled. Subjects with a minimum 12-month CD-diagnosis-free enrolment period up to the Index, and a minimum 12-month observational period after the Index were included in the analysis. Subjects were categorised into 2 groups (A: without biologics, B: with biologics) based on their first-year treatment, and then further into 4 groups: SCS without biologics (A1), No SCS nor biologics (A2), Biologics with no prior SCS (B1), Biologics with prior SCS (B2).
Results
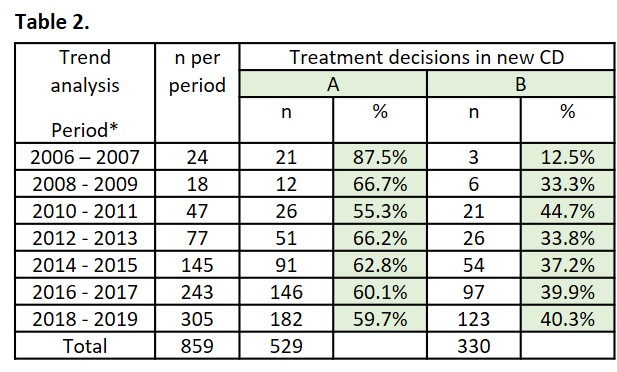
859 subjects were included. 38.4% (B: n=330/859) initiated biologics within a year since diagnosis (Table 1). Among them, 73.3% (B2: n=242/330) initiated biologics without prior SCS, so-called a top-down approach. Among the rest who were not treated with biologics within the first year, 27.2% (A1: n=114/529) received SCS. B were 7.5 years younger on average and had a higher rate of perianal symptoms (43.0%) compared to A (22.9%). Trend analyses showed that the rates of biologic adoption in the first year have been relatively stable over a decade since 2008 to 2019 (Table 2).

Conclusion
Real-world treatment decisions in newly diagnosed CD were investigated, and perianal symptoms were strongly associated with biologic therapy. Biologic adoption rates in CD seemed higher and faster in Japan compared to Europe. According to a Danish study reported by Alulis et al. (2020), 28.5% of CD patients received biologics at some point during the whole study period, of which 46.2% received within a year. These figures suggest that 13.2% of newly diagnosed CD were treated with biologics within the first year in the Danish study compared to 38.4% in our study.


