P216 Comparative Assessment C-reactive Protein Between a Point-of-Care Testing and Current Standard of Care (Immunonephelometric testing)
Lorenzon, G.(1);Marsilio, I.(1);Maniero, D.(1);Rigo, A.(1);Barberio, B.(1);Zingone, F.(1);Bahur, B.(2);Bray , K.R.(3);Savarino, E.V.(1);
(1)Padua University Hospital, Department of Surgery- Oncology and Gastroenterology, Padua, Italy;(2)ProciseDx, Department of Clinical Trials, San Diego, United States;(3)ProciseDx, Department of Clinical Development and Medical Affairs, San Diego, United States
Background
C-reactive protein (CRP) is widely used as a biomarker of inflammatory disease activity in hospitalized and non-hospitalized patients. In particular, CRP is commonly used in patients suspected to have an inflammatory bowel disease (IBD) or with a confirmed diagnosis of IBD diagnosis in order to drive the diagnostic approach, to monitor disease activity and to guide therapeutic adjustments. However, standard laboratory CRP testing (Immunonephelometric assays) present some drawbacks, including a turnaround time of 1-2 hours, and the need of specialized equipment, offices and laboratory personnel. Because of that, point-of care testing (POCT) was recently developed in order to provide results within 2 minutes from blood collection, enabling a rapid response to clinical condition.
Aim:To determine the degree of analytical correlation between a recently developed POCT (ProciseDx) using capillary whole blood and the comparative Immunonephelometric assay using serum samples.
Methods
From October to November 2020, consecutive patients hospitalized at Gastroenterology Unit, Padua University Hospital, aged > 18 years and with clinical evidence of active inflammatory disease or infection, who underwent to a standard of care CRP test (Dimension Vista – Siemens Healthineers) were included in the study (range 2.9-340 g/L). Within 1 hour from blood collection, in each patient, CRP quantitation from capillary whole blood collected by finger stick was performed using the ProciseDx CRP assay, with reportable range between 3.6-100 g/L. A Deming regression test was used to identify the correlation between the two methods.
Results
Eighty-three patients were enrolled (62.5% males with mean age ± SD: 60±18). The most common indications for hospitalisation were liver disease (34.9%), pancreatic disturbance (27.7%) and suspicious or recurrence of IBD (16.7%). ProciseDx POCT with finger prick samples required a turnaround time of 2±0.2 minutes, whereas serum samples analyzed in clinical laboratory with the reference method required a turnaround time of about 180±15 minutes (p<0.001). Overall, the correlation between the two tests was high (R squared of 0.899 (95% CI 0.916-0.968)). In particular, the correlation between the methods was even higher with CRP values between 0-100 g/L with R squared of 0.961 (95% CI 0.958-0.986). 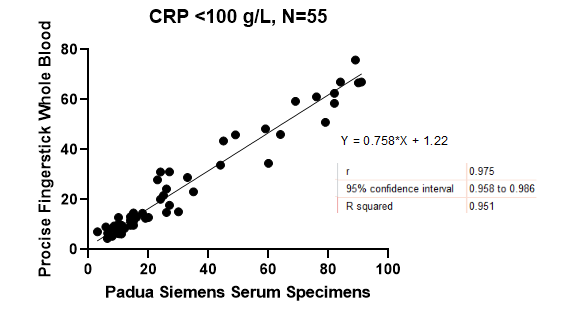
Conclusion
The ProciseDx POCT allows a more rapid and comparable accuracy of CRP assessment in hospitalized patients as compared to the standard laboratory measurement. Moreover, the ProciseDx POCT does not require specialised personnel to be performed. The use of ProciseDx POCT may improve and accelerate the decision-making approach, further reducing the resources required for CRP assessment.


