P226 Dietary patterns differ in the newly diagnosed IBD cohort with ethnicity as a key confounding variable, a case control study
Dyall, L.(1)*;Kyle, J.(2);Misra, R.(3);Clark, H.(4);Limdi, J.(5);Cooney, R.(6);Brookes, M.(7);Fogden, E.(8);Pattni, S.(9);Sharma, N.(10);Iqbal, T.(6);Munkholm, P.(11);Burisch, J.(11);Arebi, N.(3);
(1)St Mark's Hospital and Academic Institute- London- United Kingdom, Inflammatory Bowel Diseases, Slough, United Kingdom;(2)University of Aberdeen, Institute of Applied Health Sciences, Aberdeen, United Kingdom;(3)St Mark's Hospital and Academic Institute, Inflammatory Bowel Disease, London, United Kingdom;(4)University of Aberdeen, Institute of Applied Health Sciences, Abderdeen, United Kingdom;(5)Northern Care Alliance NHS Foundation Trust, Institute of Inflammation and Repair, Manchester, United Kingdom;(6)University Hospitals Birmingham, Gastroenterology, Birmingham, United Kingdom;(7)Royal Wolverhampton NHS Trust, Gastroenterology, Wolverhampton, United Kingdom;(8)Sandwell and West Birmingham Hospitals, Gastroenterology, Birmingham, United Kingdom;(9)University Leicester Hospitals, Gastroenterology, Leicester, United Kingdom;(10)Heartlands Hospital, Gastroenterology, Birmingham, United Kingdom;(11)North Zealand University Hospital, Gastroenterology, Frederikssund, Denmark;
Background
Inflammatory Bowel Disease (IBD) is driven by the interaction of the genome, environment, and diet. Our understanding of dietary risk factors is limited with a paucity of literature on specific dietary triggers for IBD. Furthermore, the role of food groups in driving inflammatory activity is conflicting compounded by independent consumption of macro- and micro-nutrients. We studied the dietary patterns of a newly diagnosed IBD cohort.
Methods
A case-controlled study with newly diagnosed IBD patients, defined as patients with symptoms, confirmatory tissue biopsy, not previously known to a gastroenterology/IBD service. Food intake within the 3 months preceding the diagnosis was measured using the Scottish Collaborative Group Food Frequency Questionnaire (FFQ). The FFQ is a validated objective tool to estimate the habitual diet. The IBD cases were matched for age and gender with controls. Principal components analysis (PCA) was used to identify dietary patterns. Factor loadings <-0.3 and >0.3 were used to select for food groups, which were infrequently and frequently consumed respectively. Binary logistic regression analysis (adjusted for age, gender, ethnicity, and total energy) was used to study differences.
Results
179 IBD cases (54 Crohn’s disease) and 144 controls were enrolled. Table 1a and 1b show the baseline population and IBD characteristics. 5 dietary patterns were identified. These were classified as: Healthy Balanced (HB), Ultra-processed (UP), Meat/Fish (MF), Pescatarian (P) and Unbalanced (UB). The food groups contributing to the patterns are listed in Table 2. Consumption of the HB and UP dietary patterns were less commonly observed in the IBD population compared to control (p<0.001, p=0.003, respectively). These observations were independent of age, gender, and total energy. No differences were observed for the MF and P diets. The UB diet was predominantly consumed by the IBD cohort (p<0.001).
When comparing the dietary pattern intake between IBD and non-IBD Caucasian groups, there was a lower consumption of the UP dietary pattern for IBD (p<0.001). This observation was not replicated in the non-Caucasian cohort.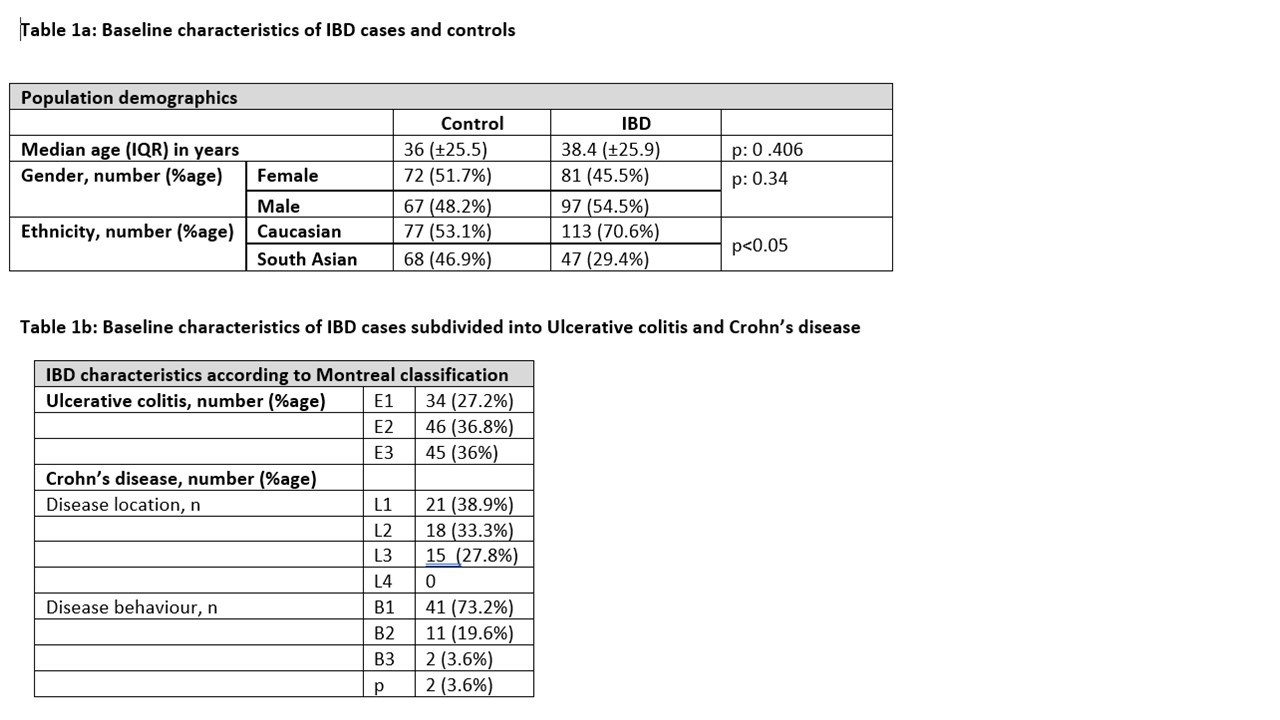

Conclusion
Newly diagnosed IBD patients follow an UB dietary habit, which is not entirely driven by an UP diet. Caucasians with IBD were less likely to adopt a UP diet than non-IBD controls. Ongoing analysis into the macro- and micro- nutrient intake compared to the nationally recommended UK guidelines, with the pro-inflammatory burden of nutrients, may offer insights into the dietary triggers for the development of IBD with potential therapeutic benefit.


