P227 Patient experiences in ulcerative colitis: conceptual model and review of patient-reported outcome measures
Kim, C.(1)*;Brown, F.L.(2);Burk, C.(3);Anatchkova, M.(4);Sargalo, N.(2);Kaushik, A.(1);
(1)Gilead Sciences, Health Economics and Outcomes Research, Foster City, United States;(2)Evidera, Patient Centered Research, London, United Kingdom;(3)HEOR Consultant, Health Economics and Outcomes Research, Laguna Beach, United States;(4)Evidera, Patient Centered Research, Bethesda, United States;
Background
Symptoms of ulcerative colitis (UC) negatively affect patients’ health-related quality of life (HRQoL). The aim of this study was to identify symptoms and impacts experienced by patients with UC, develop a conceptual model (CM) and evaluate available patient-reported outcome (PRO) measures for appropriateness for inclusion in UC clinical studies.
Methods
We conducted a landscape assessment that included: targeted literature reviews (TLRs) to identify concepts of interest and frequently used PRO measures among patients with UC; label claims searches for US and European regulatory and health technology assessment (HTA) submissions; and US and European clinical trial database searches to identify PRO measures used as primary and secondary endpoints in ongoing clinical trials. Results from the TLR informed a CM of UC. Concepts from the CM were mapped to selected PRO measures. Appropriateness of selected PRO measures for UC populations was evaluated via a gap analysis.
Results
Fifty-two symptoms and 72 impacts of UC were identified from the TLR. The most frequently reported symptoms were diarrhoea, incontinence/leaking/lack of bowel control, urgency, rectal bleeding, frequent bowel movements and fatigue. The most frequently reported impacts were anxiety, depression, inability to conduct daily activities, embarrassment and affected relationships with others. The CM grouped the 52 symptom concepts into eight dimensions, the 29 impacts proximal to UC into two dimensions and the 43 impacts distal to UC into five dimensions (Figure 1). Of the 69 PRO measures identified, eight were selected for conceptual analysis based on being disease-specific and having evidence of use in regulatory label claims, HTA submissions, as UC clinical trial endpoints and/or having US Food and Drug Administration (FDA) qualification status. The five PRO measures that covered the most concepts when mapped against the CM and included in the gap analysis were: the Inflammatory Bowel Disease Questionnaire (IBDQ); Symptoms and Impacts Questionnaire for UC (SIQ-UC); UC-PRO symptoms modules (UC-PRO-Signs and Symptoms [UC-PRO/SS] and UC-PRO-Systemic Symptoms); UC-PRO impact modules (UC-PRO-Daily Coping [UC-PRO-DC], UC-PRO-Daily Life Impact [UC-PRO-DLI] and UC-PRO-Emotional Impact [UC-PRO-EI]); and Crohn’s and Ulcerative Colitis Questionnaire (CUCQ) (Table 1). All five PRO measures had good or excellent support for content validity; the UC-PRO/SS fully met FDA PRO guidance for content validity and most psychometric properties.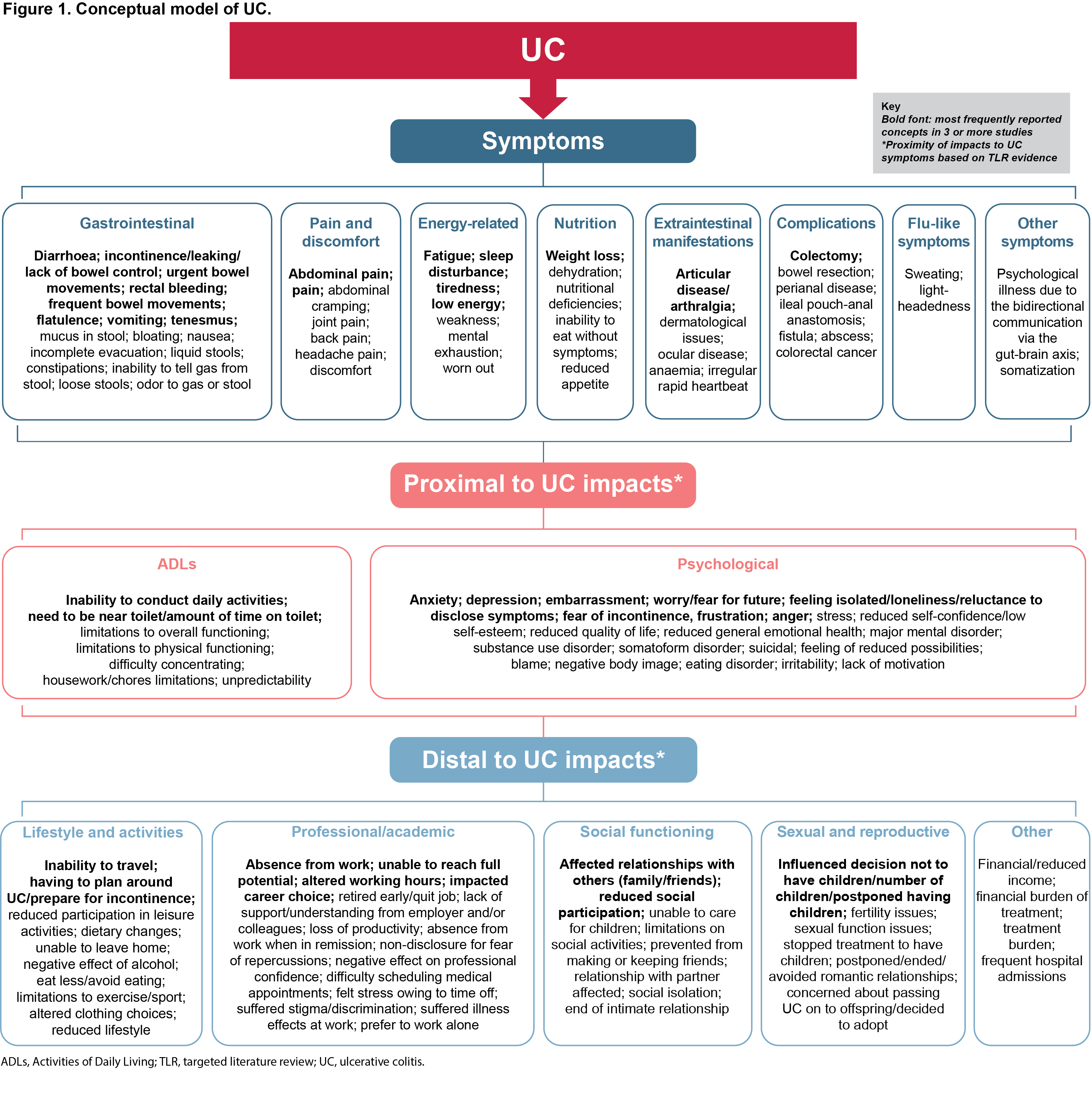

Conclusion
Based on the CM and PRO measures review, the IBDQ plus UC-PRO/SS are recommended to capture UC symptoms and IBDQ plus UC-PRO impact modules to capture proximal impacts. All PRO measures reviewed require further psychometric evaluation.


