P240 CDEIS score of 2 is optimal cut-off associated with lower risk of disease progression in early Crohn’s disease: Data from the CALM study
R. Ungaro1, R. Jordan2, C. Yzet3, P. Bossuyt4, F. Baert5, T. Vanasek6, G.R. D’Haens7, V. Wilhelmus Joustra7, R. Panaccione8, G. Novacek9, W. Reinisch9, A. Armuzzi10, O. Golovchenko11, O. Prymak11, A. Goldis12, S. Travis13, X. Hébuterne14, M. Ferrante15, G. Rogler16, M. Fumery3, S. Danese17, G. Rydzewska18, B. Pariente19, E. Hertervig20, C. Stanciu21, M. Serrero22, M. Diculescu23, L. Peyrin-Biroulet24, D. Laharie25, J.P. Wright26, F. Gomollón27, G. Irina28, S. Schreiber29, S. Motoya30, P.M. Hellström31, J. Halfvarson32, J.F. Colombel33
1Icahn School of Medicine at Mount Sinai, Department of Gastroenterology, New York City, USA, 2Icahn School of Medicine at Mount Sinai, Gastroenterology, New York, USA, 3Amiens University Hospital, Gastroenterology, Amiens, France, 4Imelda GI Clinical Research Center, Gastroenterology, Bonheiden, Belgium, 5AZ Delta Roeselare, Gastroenterology, Roeselare, Belgium, 62-nd Department of Internal Medicine, University Hospital Hradec Králové, Hradec Králové, Czech Republic, 7Amsterdam UMC, Gastroenterology, Amsterdam, The Netherlands, 8University of Calgary, Inflammatory Bowel Disease Unit, Calgary, Canada, 9Medical University of Vienna, Gastroenterology, Vienna, Austria, 10Fondazione Policlinico Universitario A. Gemelli IRCCS - Università Cattolica del Sacro Cuore, Inflammatory Bowel Disease Unit, Rome, Italy, 11Medical Clinical Investigational Center of Medical Center Health Clinic LLC, Gastroenterology, Vinnytsia, Ukraine, 12Universitatea de Medicina si Farmacie, Gastroenterology, Timisoara, Romania, 13University of Oxford, Translational Gastroenterology Unit, Oxford, UK, 14CHU of Nice- University of Nice Sophia-Antipolis, Gastroenterology and clinical Nutrition Department, Nice, France, 15University Hospitals Leuven, Gastroenterology, Leuven, Belgium, 16University Hospital Zurich, Department of Gastroenterology and Hepatology, Zurich, Switzerland, 17Humanitas University, Istituto Clinico Humanitas, Milan, Italy, 18Central Clinical Hospital of Ministry of Interior and Aministration, Warsaw and University of JK, Gastroenterology, Kielce, Poland, 19Lille University, Claude Huriez Hospital, Lille, France, 20Skane University Hospital, Gastroenterology, Lund, Sweden, 21Grigore T. Popa University of Medicine and Pharmacy, Gastroenterology, Iasi, Romania, 22North Hospital, University of Mediterranean, Hepato-Gastroenterology Department, Marseille, France, 23University of Medicine and Pharmacy ‘Carol Davila’, Gastroenterology, Bucharest, Romania, 24Hôpital de Brabois, Hépato Gastro-Entérologie-, Nancy, France, 25Hôpital Haut-Lévêque, Service d’Hépato-gastroentérologie et Oncologie Digestive, Bordeaux, France, 26Kingsbury Hospital, Gastroenterology, Cape Town, South Africa, 27Hospital Clínico de Zaragoza- IIS Aragón, Gastroenterology, Zaragoza, Spain, 28Military Medical Academy named after S.M.Kirov, Gastroenterology, Saint-Petersburg, Russian Federation, 29Kiel University, Department of Internal Medicine I, Kiel, Germany, 30Sapporo Kosei General Hospital, IBD Center, Sapporo, Japan, 31Uppsala University Hospital, Gastroenterology, Uppsala, Sweden, 32Faculty of Medicine and Health, Örebro University, Department of Gastroenterology, Örebro, Sweden, 33Icahn School of Medicine at Mount Sinai, Department of Gastroenterology, New York, USA
Background
The optimal endoscopic target in early Crohn’s disease (CD) that limits long-term disease complications is unknown.
Methods
We analysed medical records from patients who had follow-up data since the end of CALM. Patients with Crohn’s disease endoscopic index of severity (CDEIS) scores at the end of CALM were included. The primary outcome was a composite of major adverse outcomes reflecting CD progression: new internal fistula/abscess, stricture, perianal fistula/abscess, CD hospitalisation, or CD surgery since the end of CALM. We compared median CDEIS and per cent improvement from baseline CDEIS. Youden index analysis was used to identify optimal CDEIS cut-off score associated with CD progression. Kaplan–Meier and Cox regression methods were used to compare rates of progression by different CDEIS targets. Multivariable models were adjusted for age, prior surgery, and stricturing behaviour.
Results
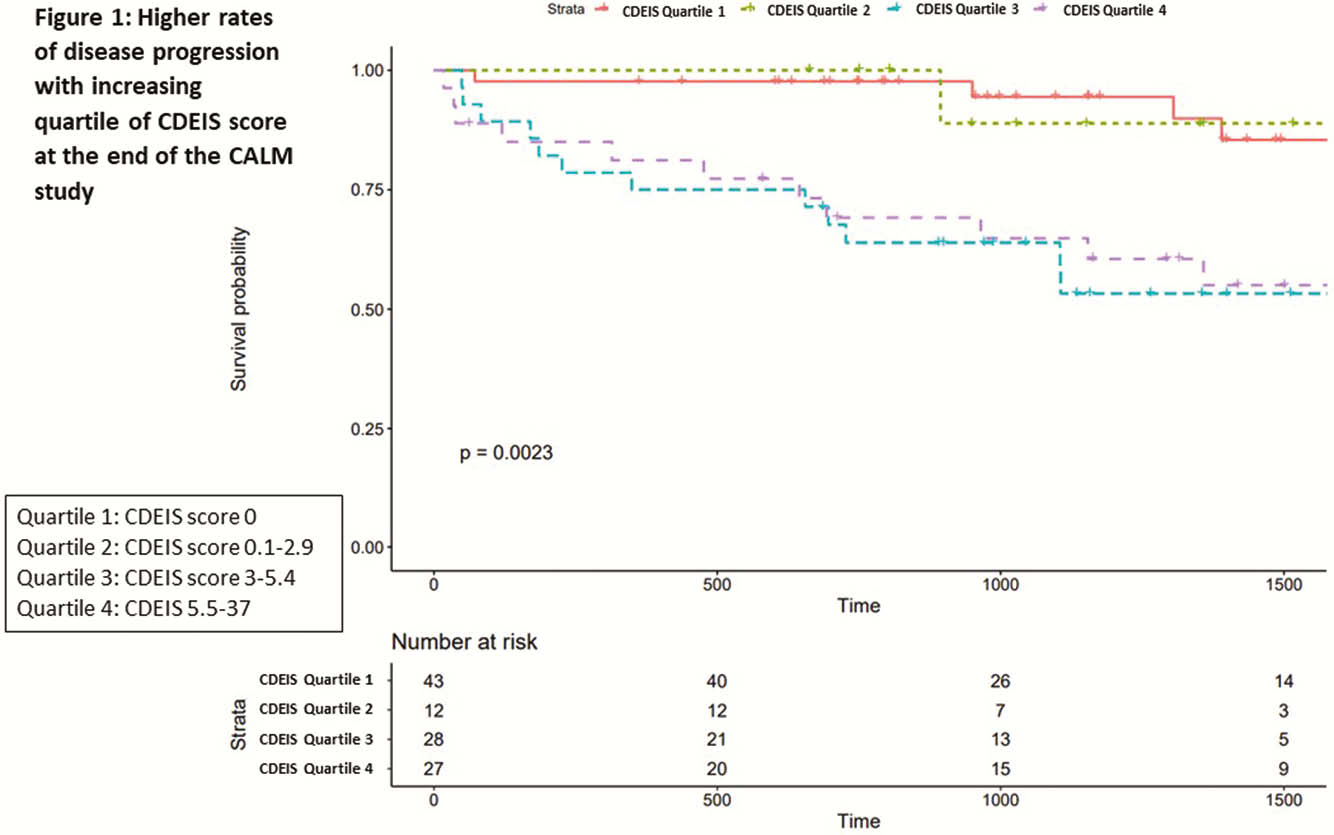
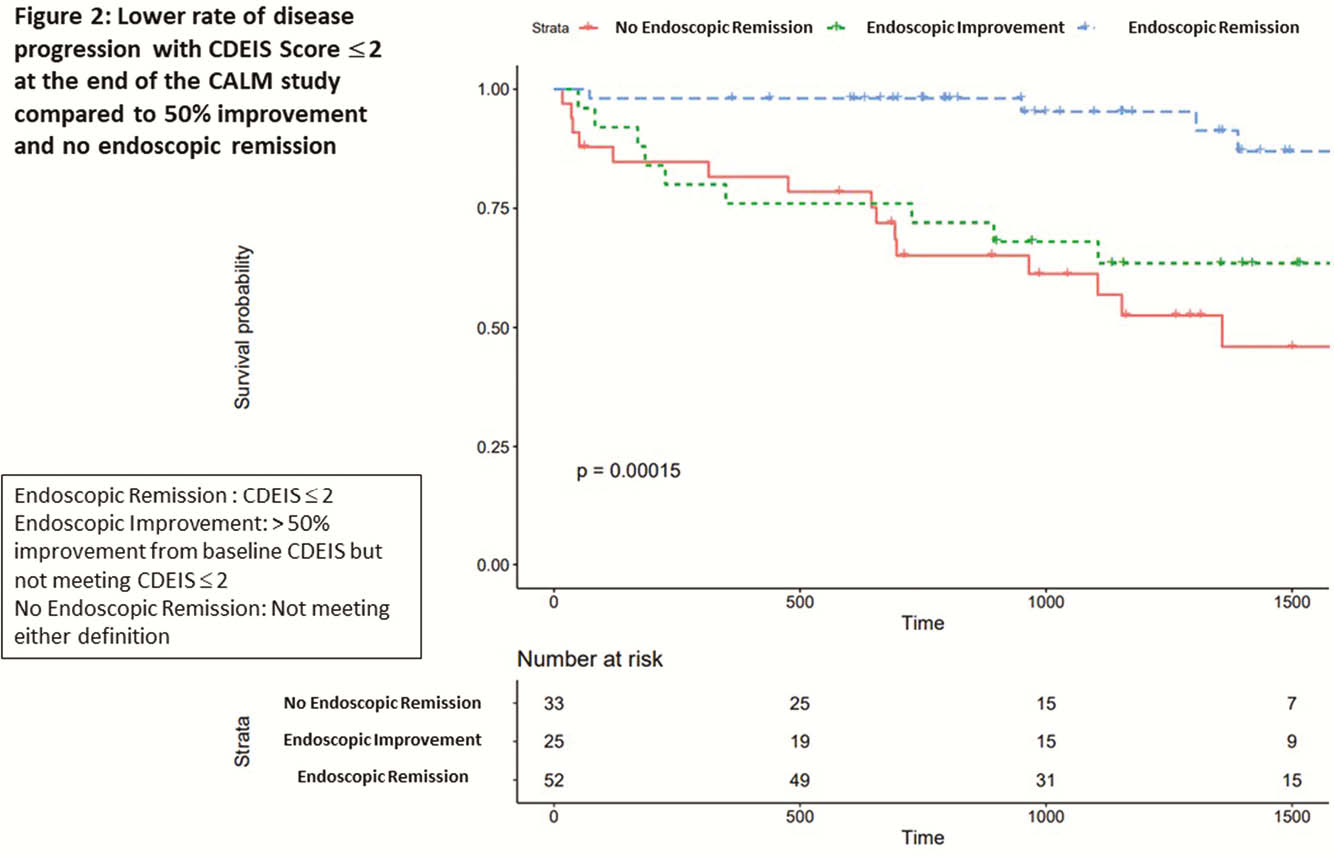
110 patients with median age 28 (IQR 22–38) years, disease duration 0.2 (0.1–0.5) years, and median follow up of 3.1 (1.9–4.4) years were included. Eleven per cent had a history of stricture, 5.5% history of surgery, and 52% were originally in the tight control arm of the CALM study. Median CDEIS score at end of CALM was 3 (0–5.4) and 32 (29%) patients had disease progression. Baseline median CDEIS score was similar between those with and without progression [10.9 (7.5–15.5) vs. 11.9 (8–17.5)]. Median CDEIS score at the end of CALM was higher among those with progression [1.3 (0–5.1) vs. 4.9 (3–9.1),
Conclusion
In early CD, a CDEIS score ≤ 2 is optimal cut-off associated with a lower risk of disease progression.


