P242 Universal exclusion of patients with Permanent Ileostomy in Crohn’s disease clinical trials: A systematic review
Vuyyuru, S.(1);Solitano, V.(1);Nguyen, T.(2);Rieder, F.(3,4);Jairath, V.(1,2,5)*;
(1)Schulich school of Medicine- Western University, Department of Medicine- Division of Gastroenterology, London, Canada;(2)Western University, Lawson Health Research Institute, London, Canada;(3)Lerner Research Institute- Cleveland Clinic Foundation, Department of Inflammation and Immunity-, Cleveland, United States;(4)Digestive Diseases and Surgery Institute- Cleveland Clinic Foundation, Department of Gastroenterology- Hepatology and Nutrition, Cleveland, United States;(5)Western University, Department of Epidemiology and Biostatistics, London, Canada;
Background
Crohn’s disease (CD) can be progressive leading to complications such as strictures and fistula requiring surgery in up to 50% of patients within 10 years of diagnosis and increased risk of permanent ileostomy (PI) formation. Approximately one third of patients with PI experience clinical recurrence within the small bowel which may need medical therapy and 16% significant recurrence requiring further surgery. Despite the physical and psychological morbidity associated with PI formation, there is a paucity of research in patients with CD and PI. We performed a systematic review to investigate whether patients with CD and PI were eligible to take part in pharmaceutical clinical trials.
Methods
MEDLINE, Embase, and the Cochrane library (CENTRAL) databases were searched from inception to April 2022 for randomized, placebo-controlled induction and maintenance trials of biologics and small molecules in adult patients with active CD. Eligibility criteria of included studies were screened by two authors independently. The primary outcome was to determine the proportion of trials that included patients with CD and a PI. Trial characteristics such as number of participants, mean age, sex, trial phase, class of drug, inclusion, or exclusion of patients with ostomy were collected.
Results
After excluding duplicates 11693 records from databases and additional sources were selected for screening. In total,144 records were assessed for full text assessment and 81 studies (induction: 58 and maintenance: 23) which enrolled 22000 participants were considered eligible (Table 1). The majority were phase 2 trials (n=41, 50.6%), 86.4% (n=70) evaluated efficacy and safety of biologics and the remaining studies (n=11, 13.6%) evaluated oral small molecules. Amongst the induction trials, 39 (67.2%) specifically stated that they excluded patients with an ostomy and the remaining studies (n=19, 32.8%) did not comment. For maintenance trials, 16 (69.6%) stated that they excluded patients with an ostomy and the remaining seven (30.4%) studies did not comment. Twenty-one induction studies and thirteen maintenance studies reported the proportion of patients with prior intestinal resection which ranged from 2.4% to 51%. No clinical trial included patients with a PI.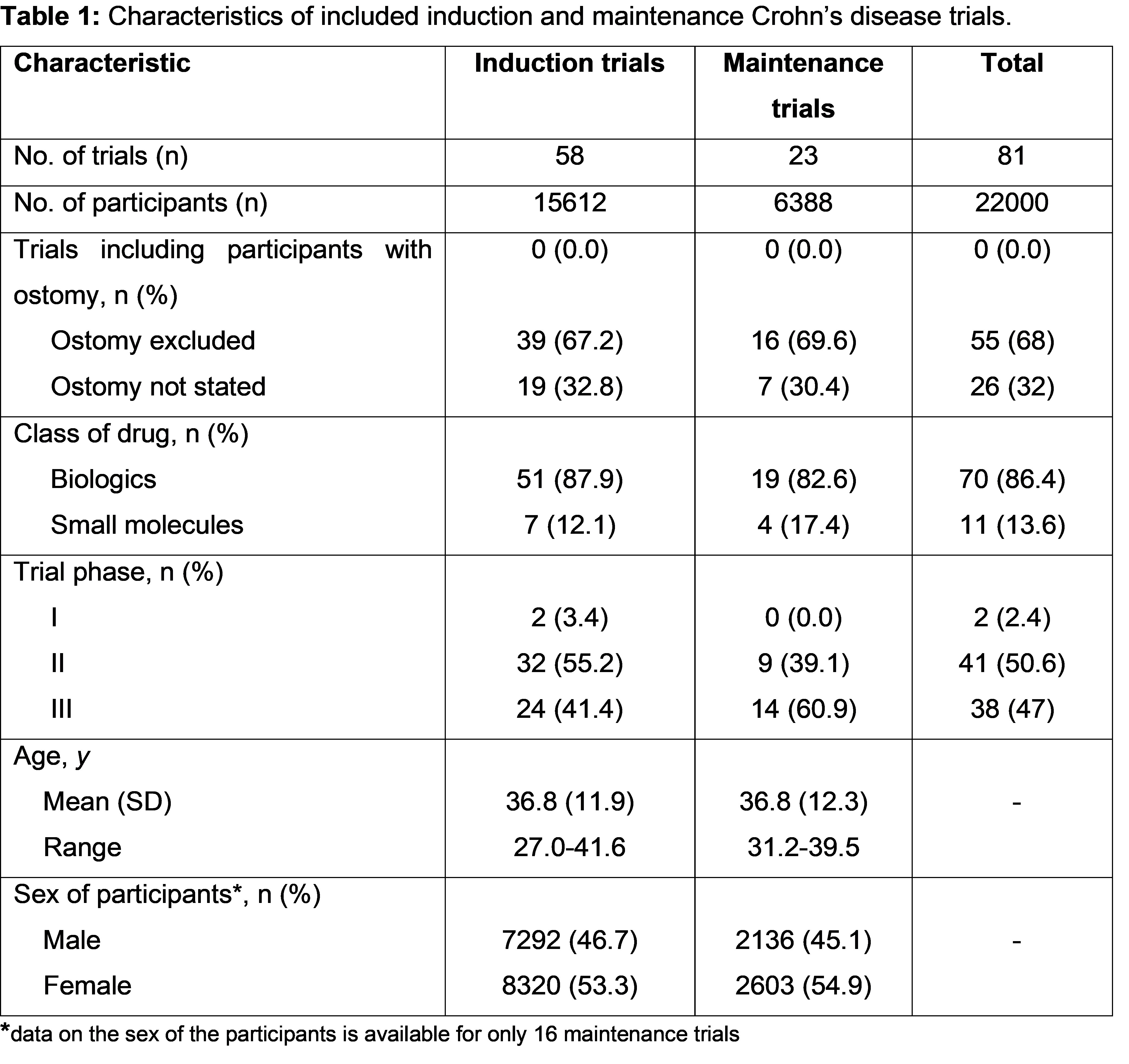
Conclusion
Patients with CD and PI have been excluded from pharmaceutical trials of biologics and small molecules to date. This group represents a high-risk patient population that have developed penetrating disease and may require medical therapy to prevent and treat recurrence. There is an urgent need to identify barriers to enrolment and develop eligibility and outcome measures that enable inclusion of patients with CD and PI into clinical trials.


