P247 Comparison of discontinuation/switching between infliximab biosimilar and originator among Inflammatory Bowel Disease patients: a real-world analysis of two Canadian cohorts
Moura, C.S.(1);Lukusa, L.(1);Wong, E.C.(2);Lakatos, P.L.(3);Afif, W.(3);Narula, N.(2);Bernatsky, S.(3);
(1)McGill University Health Centre, Centre for Outcomes Research and Evaluation, Montreal, Canada;(2)McMaster University, Department of Medicine Division of Gastroenterology, Hamilton, Canada;(3)McGill University, Department of Medicine, Montreal, Canada;
Background
Biosimilar infliximab (INF-B) has been approved in Canada for Inflammatory Bowel Disease (IBD) for over five years, but real-world descriptions of therapy persistence (discontinuation/switching) comparing INF-B to bio-originator (INF-O) are still scarce. Our objectives are to describe and compare therapy discontinuation/switching of INF-B and INF-O in IBD.
Methods
We used data from two IBD tertiary care clinics in Canada, in Montreal (QC) and Hamilton (ON). We studied biologic-naïve and biologic-experienced IBD adult initiating INF-B or INF-O between Jan. 2015 and Jul. 2020. We assessed time to discontinuation/switching through Kaplan-Meier curves (right-censoring due to loss to follow-up or end of the observation period). Reasons for discontinuation were assessed. Multivariable Cox regression models were performed to assess difference in time to discontinuation/switching between the two infliximab types. Model variables included age at infliximab initiation, sex, underlying condition (Ulcerative Colitis or Crohn's Disease), disease duration, race/ethnicity, calendar year of infliximab start, current smoking, clinical centre, and previous use of other biologics, as well as previous/current use of corticosteroids, non-biologic immunosuppressants, budesonide, or 5-aminosalicylates.
Results
We studied 306 patients followed an average of 1.4 (standard deviation, SD=0.99) years. Patients were 46.7% female, with median age at infliximab initiation of 32 (interquartile range, IQR 21 to 47) years and median disease duration of 3.5 (IQR 0.9 to 11.7) years. Age, sex, and other baseline characteristics were comparable between the two groups, though there was a trend towards more INF-B initiation in recent years (Table 1). We identified 70 discontinuation/switching events; the main reasons for discontinuation/switching were adverse events (37.1%) and secondary loss of response (28.6%). The median time to discontinuation/switching was 4.1 (95% confidence interval, CI 3.4-5.5) years among INF-O and 3.0 (95% CI 2.2-3.5) years in the INF-B group. In the multivariate model, risk of the main outcome was not clearly different between the two groups (hazard ratio, HR 1.21, 95% CI 0.64-2.32 – Table 2). Older age and corticosteroids were significantly associated with discontinuation/switching of the initial infliximab therapy.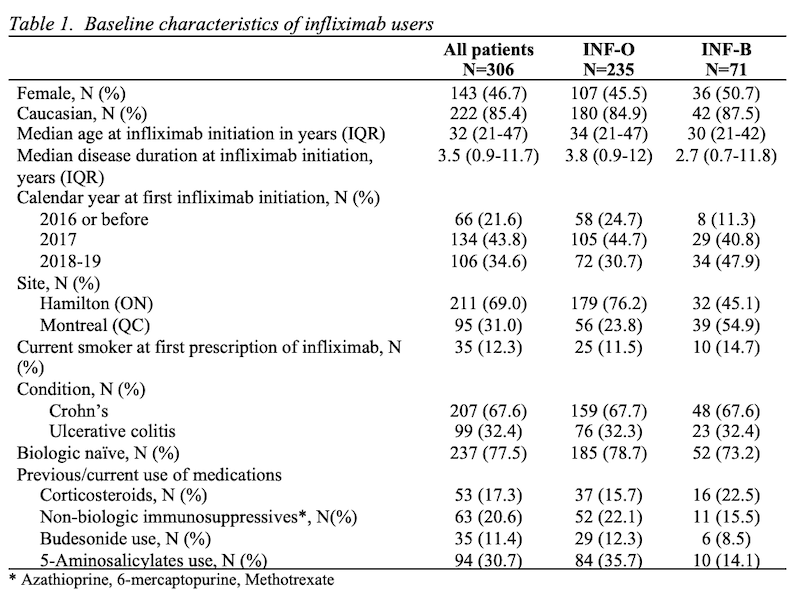

Conclusion
In this real-world analysis, we were unable to demonstrate differences in risk of discontinuation/switching among initiators of infliximab biosimilar or originator in IBD.


