P281 Evaluation of trough level and disease activity after switch from adalimumab originator to biosimilar in patients with Inflammatory Bowel Disease
Deprez, N.(1);De Somer, T.(2);Baert, D.(2);Deceuninck, M.(3);Huys, I.(2);Mattens, V.(4);Sterckx, A.(4);Vanden Branden, S.(4);Vanderstraeten, E.(2);Vandervoort, J.(4);Vanheddegem, N.(2);Dewint, P.(2);
(1)UZ GENT, Gastroenterology, Tielt, Belgium;(2)AZ Maria Middelares, Gastroenterology, Gent, Belgium;(3)AZ Sint Vincentius Deinze, Gastroenterology, Deinze, Belgium;(4)OLV Aalst, Gastroenterology, Aalst, Belgium
Background
SB5 is approved as a biosimilar to the adalimumab (ADA) originator. Bio- and efficacy equivalence, as well as comparable safety and immunogenicity have been demonstrated in Phase I and III randomised clinical trials. In this study we want to describe the trough levels and effectiveness of switch to a biosimilar in a real-life Inflammatory Bowel Disease (IBD) population.
Methods
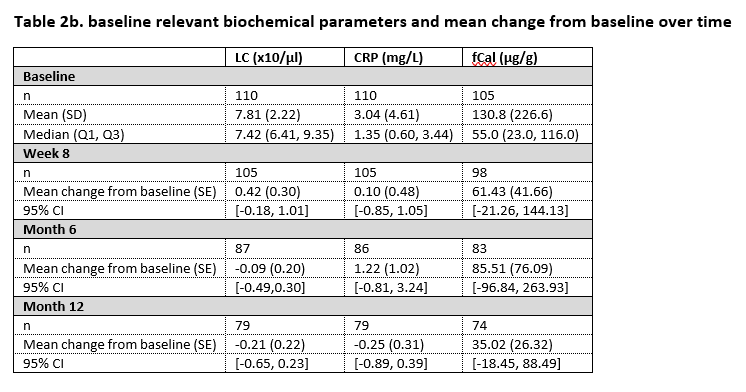
In 2 Belgian IBD centres, patients in clinical remission or stable response and treated with ADA originator were offered to enter a phase IV, interventional trial of switch to biosimilar SB5. Assessments were done at baseline, at 8 weeks, 6 and 12 months post-switch. Therapy type and dosing regimen remained unchanged the first 8 weeks; after week 8, dose adjustments could be made based on trough level and/or at the discretion of the treating physician. Trough serum ADA concentrations were measured by enzyme-linked immunosorbent assay (ELISA). The primary outcome measurement was the description of ADA trough level over time after the switch. The secondary outcome measurements were secondary loss of response (SLOR) (defined at physician’s discretion), disease activity scores and biochemical assessments (faecal calprotectin (fCal), leukocyte count (LC) and C-reactive protein (CRP)).
Results
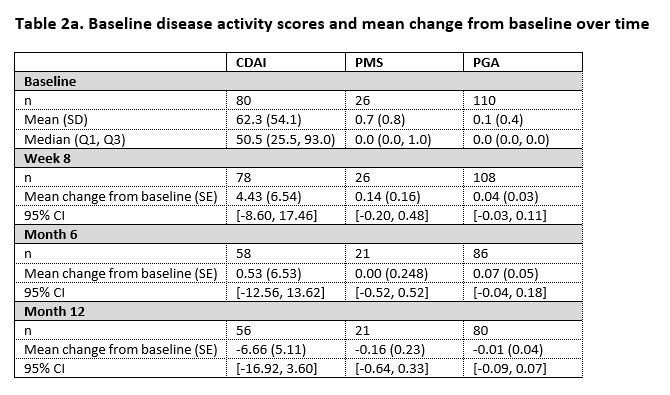
In the study, 110 patients were enrolled from whom 84 had Crohn’s disease and 26 had ulcerative colitis. By 12 months, SB5 was stopped in 5 patients because of high ADA antidrug antibodies at both baseline and at week 8. Nine patients presented with SLOR of whom 3 discontinued treatment with SB5, the remaining 6 patients received treatment optimisation. Table 1 displays the core results concerning the ADA trough levels over time. Table 2a and 2b display disease activity scores and biochemical parameters at the different time points.

Conclusion
In this pragmatic, interventional phase IV trial, ADA trough levels remain within the therapeutic range after switch from originator to SB5. The proportion of patients in our study with SLOR is in line with what is described in literature. In patients persisting on SB5, no change in disease activity over time is observed, based on both disease activity scores and biochemical parameters.


