P299 Disability in newly diagnosed patients with Crohn´s Disease: initial results from the prospective CROCO (Crohn´s Disease Cohort) Study
Tinoco da Silva TorresMD- PhD, J.(1,2)*;Ellul, P.(3);Vieujean, S.(4);Ordas, I.(5);Burisch, J.(6);Mocanu, I.(7);Duricova, D.(8);Rodríguez-Lago, I.(9);Buisson, A.(10);Goldis, A.(11);Hernandez, V.(12);Arebi, N.(13);Kaimakliotis, I.(14);Nachury, M.(15);Fumery, M.(16);Allocca, M.(17);Pedersen, N.(18);Barberio, B.(19);Shaji, S.(20);Guedes, A.(21);Ribeiro, R.(21);Ungaro, R.(22);Lambert, J.(23);Colombel, J.F.(22);
(1)Surgical Department- Gastroenterology Division, Hospital Beatriz Ângelo, Loures, Portugal;(2)Hospital da Luz, Division of Gastroenterology, Lisbon, Portugal;(3)Mater dei Hospital, Gastroenterology, Msida, Malta;(4)University Hospital CHU of Liège, Department of Gastroenterology, Liège, Belgium;(5)Hospital Clinic Barcelona, Department of Gastroenterology, Barcelona, Spain;(6)Copenhagen University Hospital - Amager and Hvidovre, Gastrounit medical division, Hvidovre, Denmark;(7)Hospital Garcia da Orta, Department of Gastroenterology, Almada, Portugal;(8)IBD Clinical and Research Clinic- ISCARE, Gastroenterologie, Prague, Czech Republic;(9)Hospital Universitario de Galdakao, Department of Gastroenterology, Galdakao, Spain;(10)CHU Clermont - Ferrand, IBD Unit, Clermont-Ferrand, France;(11)Algomed Policlinic, Gastroenterologie, Timisoara, Romania;(12)Hospital Alvaro Cunqueiro, Gastroenterologie, Vigo, Spain;(13)St Mark's Hospital, Department of IBD, London, United Kingdom;(14)American Gastroenterology Center, Gastroenterologie and Hepatologist, Strovolos, Cyprus;(15)Claude Huriez Lille University, Gastroenterologie and Hepatologist, Lille, France;(16)CHU Amiens-Picardie Hôpital Sud, Department of Hepato - Gastroenterology, Amiens, France;(17)Ospedale San Raffaele, Department of Gastroenterology, Milan, Italy;(18)Slagelse Hospital, Gastroenterologie, Slagelse, Denmark;(19)University of Padua, Division of Gastroenterology- Department of Surgery- Oncology and Gastroenterology, Padua, Italy;(20)Hull University Teaching Hospitals, IBD Research Unit- Department of Gastroenterology, Hull, United Kingdom;(21)Hospital da Luz Learning Health, Investigation Center, Lisbon, Portugal;(22)Mount Sinai Hospital, Department of Gastroenterology, New York, United States;(23)Centre of Research in Epidemiology and Statistics- UMR 1153- Inserm, ECSTRRA Unit, Paris, France;
Background
CD is classically seen as a progressive disease leading to bowel damage and disability. Disability has been proposed by the Spirit-IOIBD consensus1 as an endpoint in disease modification trials. However, there is a paucity of data regarding disability in CD patients at diagnosis.
Methods
The “Crohn´s Disease Cohort Study” (CROCO) is an ongoing prospective cohort aiming to study the longitudinal evolution of bowel damage and disability following diagnosis of CD. All patients are included within 12 months following CD diagnosis and will be followed over 5 years with serial assessments of bowel damage and disability. At recruitment patients completed an IBD disability score questionnaire (DS),2 comprising several domains: mobility, self-care, major daily life activities, gastrointestinal-related problems, mental health and interaction with the environment. We here describe the baseline DS and its association with disease features, disease activity score (Harvey-Bradshaw Index), and biomarkers of disease activity, on the first patients recruited into CROCO.
Results
Ninety-four patients were recruited, of which 66 filled at least 80% of the questions in the DS questionnaire; of those 55% were male and median age at diagnosis was 30.6 [IQR 24.2;38.8]. The baseline visit occurred at a median time of 6 months [IQR 2.6;9.2] after CD diagnosis. Ileal involvement (L1/L3) was reported in 82% of patients; 68% had B1 phenotype, and 26% presented extra-intestinal manifestations. Overall, 32% of patients had been hospitalised, and 6% had a history of CD-related surgery. Regarding medication [from diagnosis to baseline], 55% received steroids, 56% immunosuppressants, and 65% Anti-TNF therapy. The median DS was 79 [IQR 67;108.2] (range: 49;198); only 2 patients (3%) patients presented DS>162 (the central value of the DS), indicating no or mild disability. No correlations were found between clinical or analytical biomarkers of disease activity at the time of recruitment (Table 1). Associations between disease features and DS are displayed in Table 2. A history of CD-related hospitalisation was associated with a significantly higher DS (103 [IQR 79;157] versus 76.5 [65.5;97], p=0.013). No other associations were observed.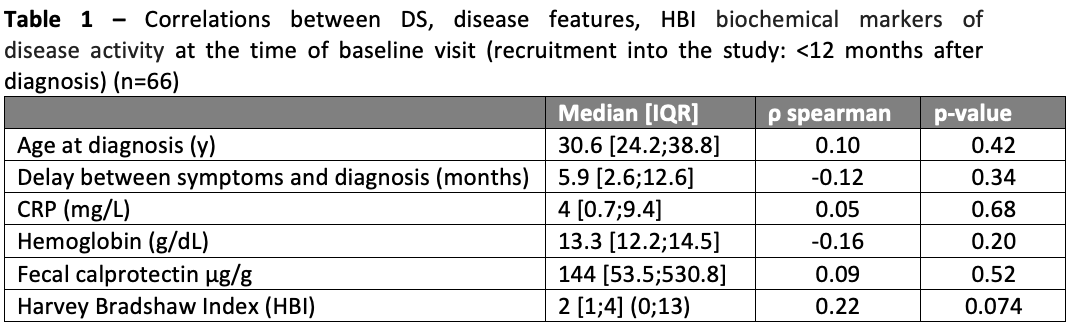

Conclusion
In a cohort of newly diagnosed CD patients, disability was mostly mild and independent of disease activity, disease location or behaviour; around 1/3 required hospitalisation within the first year of diagnosis, which was associated with increased disability. These data add to the growing concept that newly diagnosed disease represents a window of opportunity for intervention.


