P318 Protein FingerPrint assays reflecting neutrophil activity, mucosal damage, and tissue fibrosis are surrogate biomarkers for mucosal healing in pediatric Crohn’s disease
Mortensen, J.(1)*;Focht, G.(2);Pehrsson, M.(1);Griffiths, A.M.(3);Alexdóttir, M.S.(1);Quteineh, A.(2);Church, P.C.(3);Baldassano, R.N.(4);Silverstein, J.(5);Karsdal, M.A.(1);Turner, D.(2);
(1)Nordic Bioscience A/S, Biomarkers & Research, Herlev, Denmark;(2)Shaare Zedek Medical Center, Pediatric Gastroenterology and Nutrition Institute, Jerusalem, Israel;(3)Hospital for Sick Children, Division of Gastroenterology, Toronto, Canada;(4)The Children's Hospital of Philadelphia, Division of Gastroenterology- Hepatology- and Nutrition, Philadelphia, United States;(5)Harvard Medical School, Division of Gastroenterology- Hepatology and Nutrition Instructor of Pediatrics, Boston, United States;
Background
Mucosal healing (MH) has become a treatment goal for inflammatory bowel disease (IBD), especially for ulcerative colitis. However, MH is less feasible to assess frequently in patients with Crohn’s disease (CD). In this study, we investigated Protein Fingerprint Assays (PFA) of collagen degradation and formation, and neutrophil and macrophage activity markers in the ImageKids study as non-invasive markers of MH.
Methods
This is a proteomics planned sub-study of the multi-center prospective ImageKids study which included serum samples from children with CD (n=178) undergoing MRE and ileocolonoscopy concurrently (ImageKids study: NCT01881490). Serum from healthy age and gender-matched controls were obtained from BioIVT (n=83). PFA panel for mucosal damage (C1M, C3M, C4M, C6M, ELP-3), Immune cell activity panel (CPa9-HNE, VICM, C4G), and tissue fibrosis panel (PRO-C1, PRO-C3, PRO-C4, PRO-C5, PRO-C6, PRO-C11, PRO-C22) were determined. MH was classified as having an SES-CD score of 0 and global radiologist assessment of inflammation in the MRE of 0, using a visual analogue scale (VAS) 0-100mm, and mucosal damage (MD) was defined as having SES-CD or MRE VAS>0. Spearman rho correlations, one-way ANOVA with Tukey correction, and receiver operator characteristics (ROC) curves were applied for statistical analysis.
Results
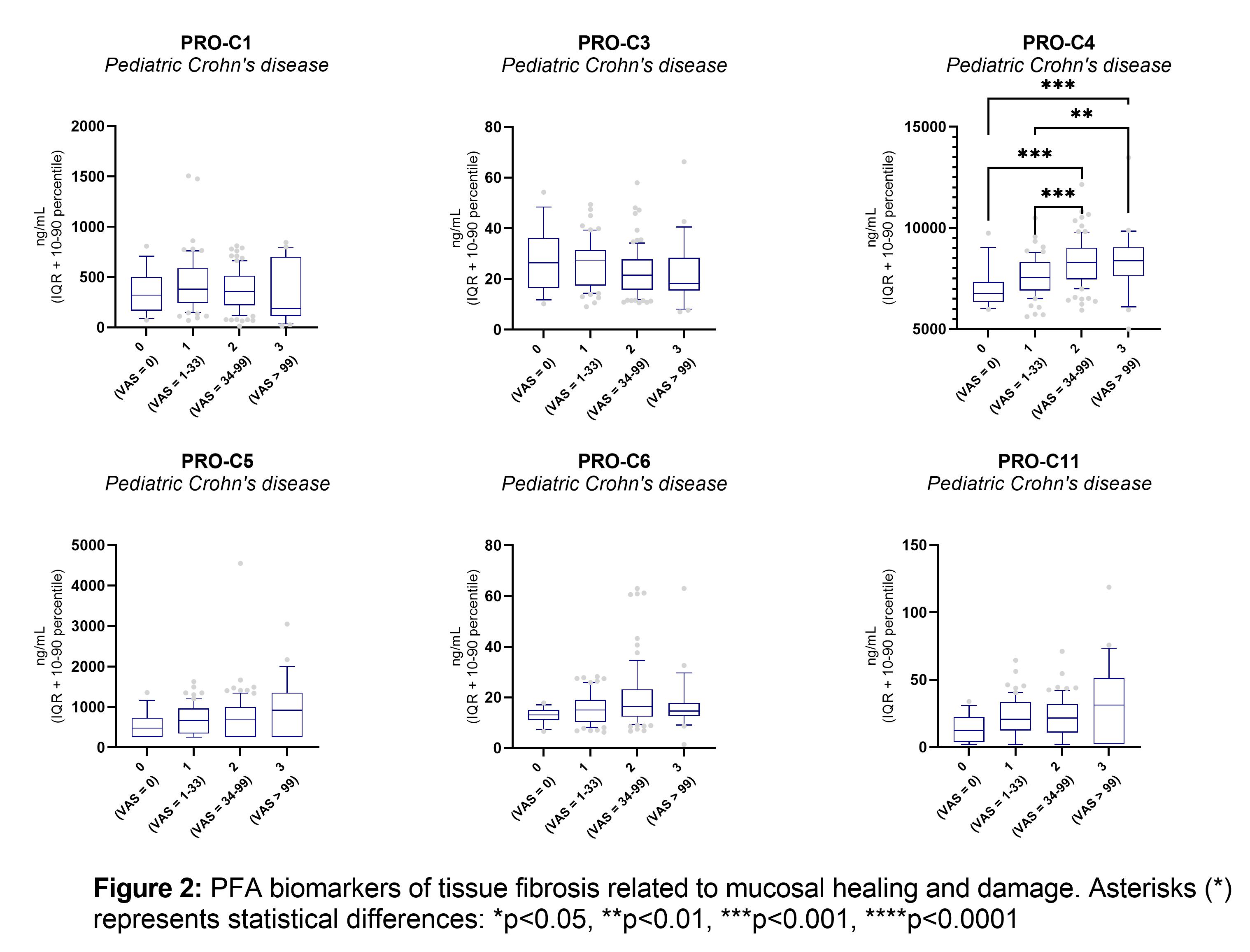
Some of the previous biomarkers demonstrated to correlate with the SES-CD score and ulcer size (C1M, C3M, C6M, PRO-C4, VICM, PRO-C1, PRO-C3). CPa9-HNE, ELP-3, and PRO-C22 also correlate with the SES-CD score (table 1). The combined SES-CD and MRE VAS score of MH correlated positively with CPa9-HNE (r=0.30, p<0.0001), VICM (r=0.39, p<0.0001), C1M (r=0.29, p=0.0001), C3M (r=0.22, p=0.0022), C6M (r=0.35 p<0.0001), PRO-C4 (r=0.2, p<0.0001), PRO-C22 (r=0.32, p<0.0001), (figure 1 and 2) and showed significantly lower serum levels in pCD patients with MH compared to patients with mild, moderate, or severe mucosal damage (p<0.001) (figure 2). PRO-C3 correlated negatively with the combined SES-CD and MRE VAS score (r=-0.17, p=0.0211). These PFA biomarkers were combined in a multivariate regression model, demonstrating increased accuracy to identify patients in MH healing compared to the individual biomarkers (p<0.001, MH vs. MD: AUC=0.82 [CI:0.69-0.95), MH+Mild MD vs. moderate+severe MD: AUC=0.77 [0.70-0.84], (figure 3).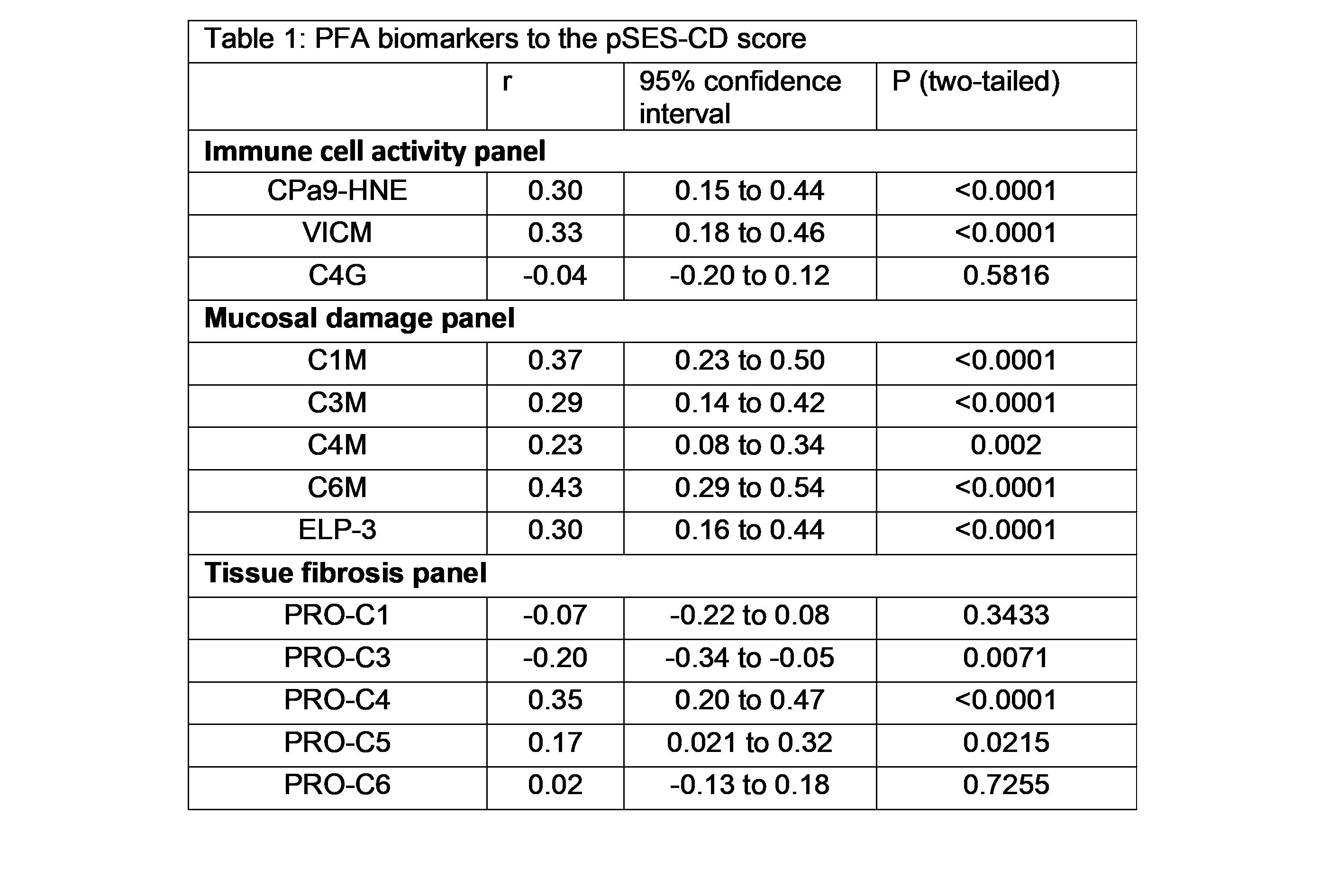


Conclusion
PFA biomarkers of Neutrophil activity (CPa9-HNE), Macrophage activity (VICM), mucosal damage (C1M, C3M, C4M, C6M, ELP-3), and tissue fibrosis (PRO-C3, PRO-C22) are potential surrogate biomarkers for identifying pCD patients with mucosal healing and for monitoring of mucosal damage and stenosis.


