P324 Predictors of corticosteroid-free remission after initiation of ustekinumab for ulcerative colitis: a real-world, multicenter cohort study in the United States
Dalal, R.(1);Esckilsen, S.(2);Barnes, E.(3);McClure, E.(1);Goodrick, H.(1);Allegretti, J.(1);
(1)Brigham and Women's Hospital- Harvard Medical School, Gastroenterology- Hepatology and Endoscopy, Boston, United States;(2)University of North Carolina at Chapel Hill, Medicine, Chapel Hill- NC, United States;(3)University of North Carolina at Chapel Hill, Gastroenterology and Hepatology, Chapel Hill- NC, United States
Background
Clinical trial data has demonstrated the efficacy of ustekinumab (UST) for the treatment of ulcerative colitis (UC), however real-world clinical outcomes data are limited. We therefore performed a real-world multicenter cohort study to identify predictors of corticosteroid-free clinical remission after initiation of ustekinumab for UC.
Methods
This is a retrospective cohort study of adult UC patients initiating UST between 1/1/2016 and 11/1/2020 at three large IBD referral centers in the United States. Patients with prior colectomy and those receiving UST for primarily non-UC indications were excluded. Electronic health records were reviewed to obtain clinical data. Independent variables present at UST induction included demographics, disease duration, extraintestinal manifestations, current/prior IBD medications, substance use (cigarettes, cannabis, or opioids), last endoscopic extent/severity, last serum albumin and C-reactive protein (within 3 months prior to induction), and daily bowel frequency. The primary outcome was corticosteroid-free remission (i.e. simple clinical colitis activity index [SCCAI] or 9-point Mayo score of <3) 12-16 weeks after induction. Secondary outcomes included clinical response (reduction of SCCAI or Mayo score by >2 points from baseline) 12-16 weeks after induction and UST failure (i.e. UST discontinuation or colectomy due to uncontrolled disease) within 52 weeks after induction. Multivariable logistic regression was used to identify factors associated with remission.
Results
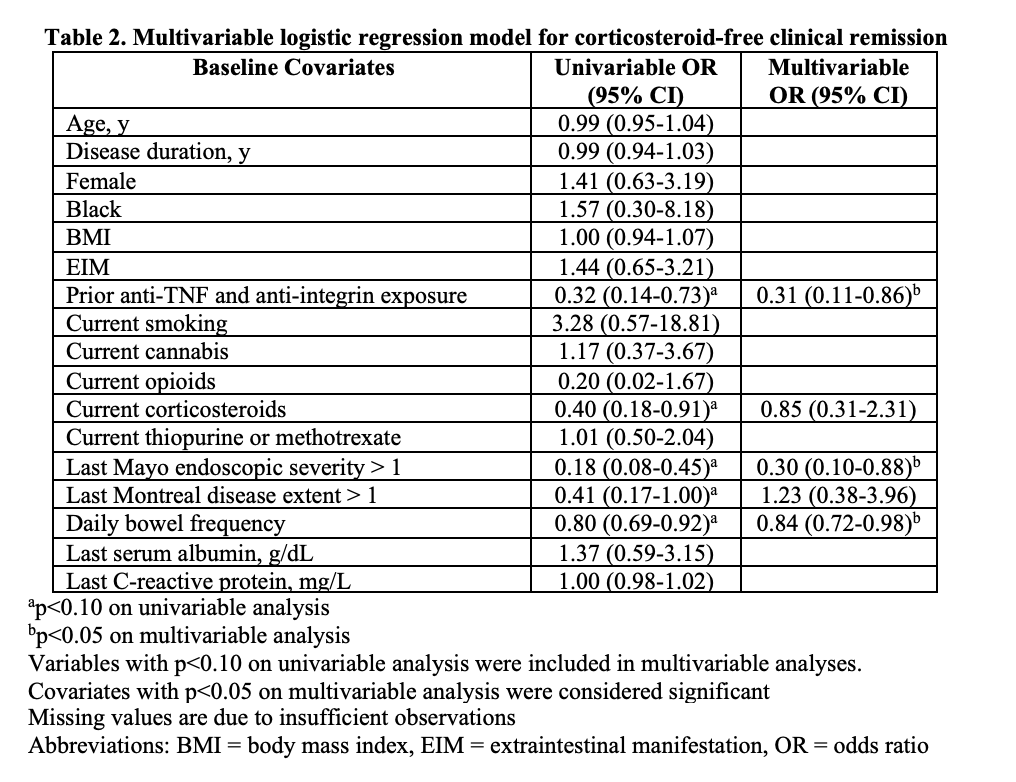
We identified 108 UC patients who initiated UST. Median age at induction was 39 years (IQR 30-56 years), 91.7% (99/108) of patients had prior anti-TNF exposure, and 60.2% (65/108) had prior anti-TNF and anti-integrin exposure (Table 1). Of 101 patients with available clinical follow-up data, 39.6% achieved remission and 51.5% had clinical response 12-16 weeks after induction. UST failure occurred in 41.1% (23/56 with sufficient follow-up) within 52 weeks. Adverse events were reported in 3.0% (3/101; rash, urinary tract infection, and C. difficile infection). After multivariable logistic regression, prior anti-TNF and anti-integrin exposure (OR 0.31, 95% CI 0.11-0.86), Mayo endoscopic severity >1 (OR 0.30, 95% CI 0.10-0.88), and bowel frequency (OR 0.84, 95% CI 0.72-0.98) were inversely associated with remission (Table 2).
Conclusion
In this multicenter cohort, nearly 40% of UC patients achieved corticosteroid-free remission 12-16 weeks after UST induction. Prior exposure to both anti-TNF and anti-integrin therapies, endoscopic severity, and daily bowel frequency were associated with failure to achieve remission. Prospective studies are needed to optimize management strategies for UC patients with failure of two biologic classes.


