P353 Pan-enteric capsule endoscopy enables assessment of mucosal healing after biologic treatment initiation in Crohn’s disease
Volkers, A.(1);Bosuyt, P.(2);de Jong, J.(1);D'Haens, G.(1);Löwenberg, M.(1);
(1)Amsterdam UMC- location AMC, Gastroenterology and Hepatology, Amsterdam, The Netherlands;(2)Imeldaziekenhuis, Gastroenterology and Hepatology, Bonheiden, Belgium
Background
Pan-enteric capsule endoscopy (pan-CE) visualizes the entire gastrointestinal tract and provides an attractive alternative to conventional ileocolonoscopy to evaluate luminal disease activity in Crohn’s disease (CD). An earlier study showed that pan-CE is feasible, safe and well tolerated by CD patients. The aim of the present study was to assess the Sensitivity TO measure Change (STOC) in mucosal disease activity using pan-CE in CD.
Methods
Patients with CD and active disease based on symptoms (i.e. Crohn’s disease activity index (CDAI) >150) and inflammatory biomarkers (i.e. C-reactive protein (CRP) >5 mg/L and/or fecal calprotectin (fCal) levels >250 mg/kg) underwent pan-CE, using the second-generation Pillcam colon capsule, prior to and 8-12 weeks after treatment initiation with infliximab, adalimumab or vedolizumab. Luminal disease activity was assessed using the Crohn’s disease endoscopic index of severity (CDEIS) and the simple endoscopic score for Crohn’s disease (SES-CD), expanded with two segments for the jejunum and pre-terminal ileum. Sensitivity to detect mucosal change was assumed if the standardized effect size was >0.8. Correlations between the evolution in CDAI, CRP and fCal with endoscopic activity scores before and after treatment initiation were calculated.
Results
Twenty-eight patients underwent pan-CE of whom two withdrew consent and four did not start biologic therapy. Twenty-two patients (78.6%) started biologic treatment and underwent pan-CE twice. Median duration between treatment initiation and pan-CE was 11 weeks (IQR: 9.8-12 weeks). Half of patients (11/22) were female and the median age was 24 years (IQR=22-38 years). The standardized effect size was 1.11 for CDEIS and 1.36 for SES-CD. Median CDEIS scores decreased from 6.8 (IQR=4.6-11.2) to 3 (IQR=0.9-6.0, p=0.01). Median SES-CD scores decreased from 15.5 (IQR=9.8-22.5) to 6 (IQR=2.8-11.3, p=0.001). SES-CD score per segment decreased significantly for colon segments, not for small bowel segments (figure 1). The difference in CDAI, CRP and fCal outcomes before and after treatment did not correlate with the change in CDEIS and SES-CD.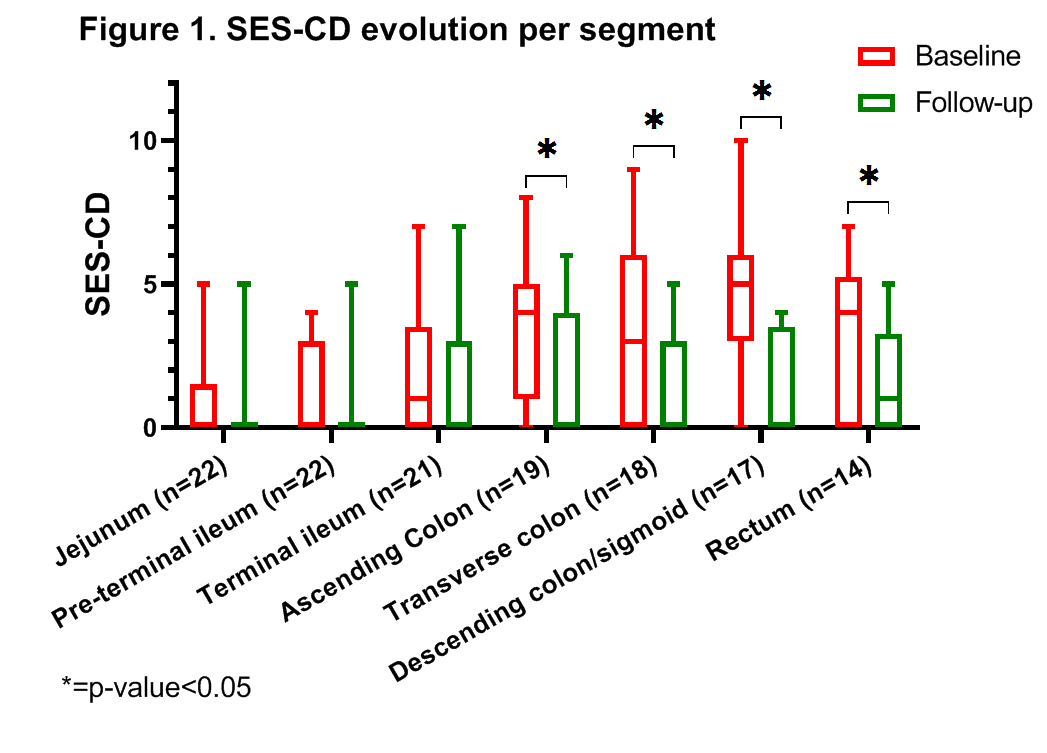
Conclusion
Pan-CE is useful to assess luminal disease activity in CD patients who embark on biological treatment. Pan-CE is sensitive to monitor mucosal healing outcomes and might therefore replace ileocolonoscopy for this indication in CD patients.


