P354 Application of the clinical decision support tool to predict treatment outcomes in Crohn’s Disease patients treated with vedolizumab subcutaneous formulation
Dulai, P.(1)*;Lindner, D.(2);Agboton, C.(3);Alric, H.(4);Bamias, G.(5);Peyrin-Biroulet, L.(6);
(1)Northwestern Medical Group, Northwestern Medicine Digestive Health Center, Chicago, United States;(2)Takeda, Takeda, Zurich, Switzerland;(3)Takeda, Takeda, Cambridge, United States;(4)Assistance Publique-Hôpitaux de Paris, Service d'hépato-gastro-entérologie et endoscopies digestives- Hôpital Européen Georges Pompidou, Paris, France;(5)University of Athens, Sotiria Hospital, Athens, Greece;(6)Nancy University Hospital, Gastroenterology, Nancy, France;
Background
There is a need to identify Crohn’s Disease (CD) patients who will obtain most clinical benefit from treatment with vedolizumab (VDZ), an anti-α4β7 integrin inhibitor for the treatment of ulcerative colitis and Crohn’s Disease (CD). Previously, a clinical decision support tool (CDST) has been developed and validated to guide treatment decisions in CD patients (pts) treated with intravenous VDZ.1 In this study, we aimed to test the ability of the CDST to predict clinical outcomes in CD pts receiving maintenance treatment with the subcutaneous (SC) formulation of VDZ.
Methods
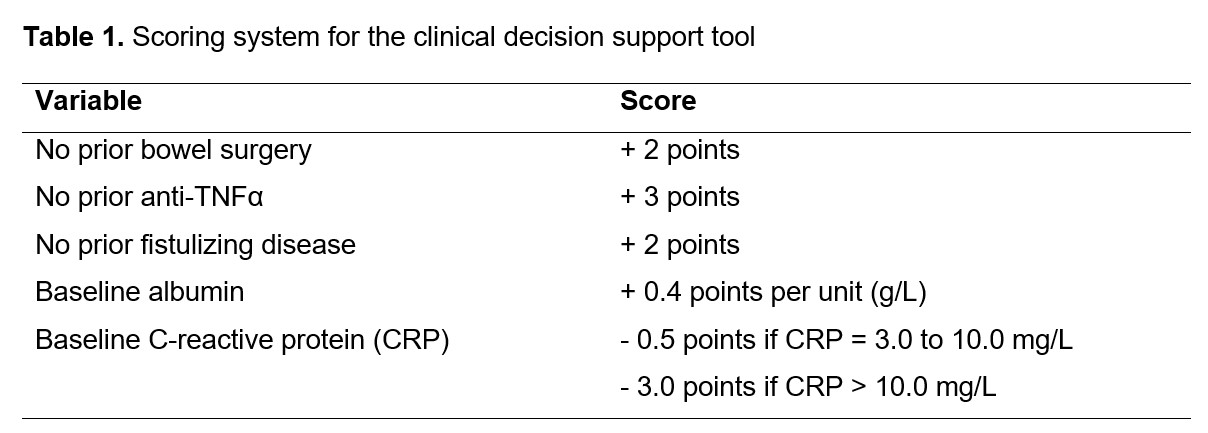
Pts with moderate to severe CD receiving maintenance treatment with VDZ SC in the VISIBLE 2 study were categorized using the CDST into low (≤13 points), intermediate (>13 and ≤19 points) and high response probability groups (>19 points) based on the scoring system detailed in Table 1. Clinical outcomes evaluated included clinical remission: CD Activity Index (CDAI) ≤ 150 at Week 6, 14, 30, and 52, and enhanced clinical response: decrease from baseline in CDAI score ≥ 100 points at Week 52. All outcomes were evaluated as observed.
Results
There were 275 pts who completed induction treatment with VDZ 300 mg IV and continued into maintenance treatment with VDZ 108 mg SC until Week 52. Using the CDST, at baseline, 14 pts (5.1%) had a low, 98 (35.6%) had an intermediate, and 163 (59.3%) had a high probability of treatment response with VDZ. Due to the low number of pts in the low probability group, the low and intermediate groups were combined. At Week 6, 99 pts (61.1%) in the high probability group had achieved clinical remission vs 38 pts (33.9%) in the low/intermediate probability group (Fig 1). Rates of clinical remission in the high probability group continued to exceed those of the low/intermediate group through until Week 52 when 89 pts (86.4%) in the high probability group were in clinical remission vs 43 pts (79.6%) in the low/intermediate probability group (Fig 1). In the high probability group, 97 pts (94.2%) had an enhanced clinical response at Week 52 compared with 46 pts (85.2%) in the low/intermediate group.
Conclusion
Compared with the low/intermediate group, pts categorized by the CDST as having the highest probability of treatment response to VDZ achieved clinical remission sooner, as early as Week 6. These findings provide further confirmation of the CDST as a means of identifying pts with CD likely to benefit from treatment with VDZ in either IV or SC formulations.
References: 1. Dulai PS et al. (2018) Gastroenterology 155:687-695.e10.