P366 CRP levels and PMS as early predictors of clinical and endoscopic outcomes in adult patients with moderately-to-severely active UC treated with tofacitinib: a post hoc analysis of OCTAVE Induction 1 and 2
M.C. Dubinsky1, D.P. Hudesman2, F. Steinwurz3, N. Kulisek4, L. Salese4, J. Paulissen5, C. Su4, D. Ponce de Leon6, F. Magro7
1Icahn School of Medicine at Mount Sinai, New York, New York, USA, 2New York University, New York, New York, USA, 3Unit of Inflammatory Bowel Disease, Hospital Israelita Albert Einstein, São Paulo, Brazil, 4Pfizer Inc., Collegeville, Pennsylvania, USA, 5Syneos Health, Morrisville, North Carolina, USA, 6Pfizer Inc., Lima, Peru, Peru, 7University of Porto and Centro Hospitalar São João, Porto, Portugal, Portugal
Background
Tofacitinib is an oral, small-molecule JAK inhibitor for the treatment of ulcerative colitis (UC). In a Phase 2 study, changes from baseline in C-reactive protein (CRP) levels as early as Week 4 and partial Mayo scores (PMS) as early as Week 2 were shown to correlate with clinical and endoscopic outcomes at Week 8 in patients with UC receiving tofacitinib.1 We aimed to determine whether CRP levels and/or PMS can be early predictors of clinical or endoscopic outcomes at Week 8 in OCTAVE Induction 1 and 2 (NCT01465763; NCT01458951).2
Methods
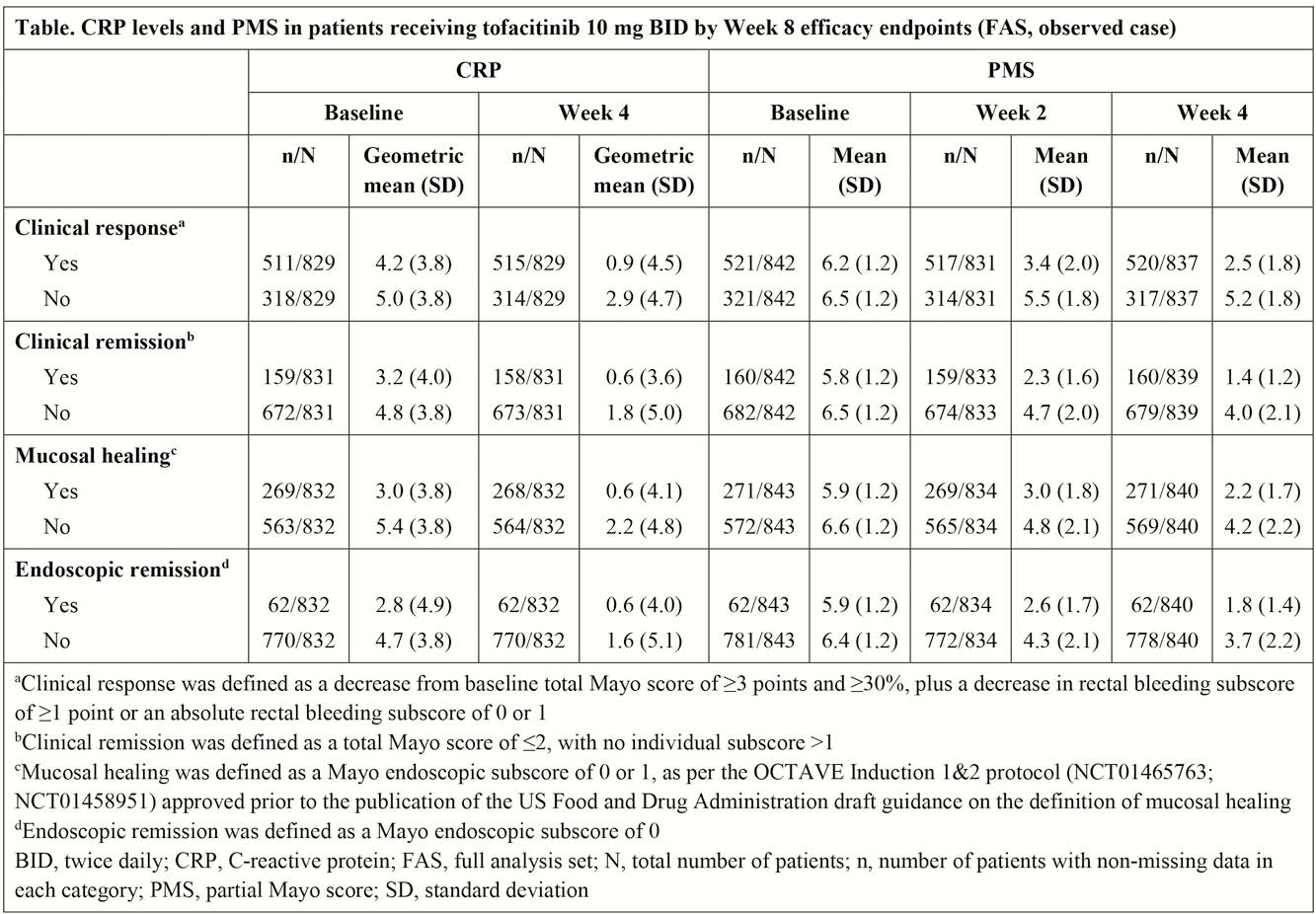
In OCTAVE Induction 1 and 2, patients received placebo or tofacitinib 10 mg twice daily (BID) for 8 weeks. Mean CRP levels at baseline and Week 4, and PMS at baseline and Weeks 2 and 4, were analysed by secondary endpoint (clinical response, clinical remission, mucosal healing and endoscopic remission) status at Week 8. Univariate logistic regression analyses were performed to evaluate if CRP levels (log-transformed) or PMS were associated with these endpoints.
Results
In patients treated with tofacitinib 10 mg BID, numerically greater decreases from baseline were observed in CRP levels at Week 4 in patients who achieved a clinical response at Week 8 vs. those who did not achieve a clinical response (Table). Similarly, numerically greater decreases from baseline were observed in PMS at Weeks 2 and 4 in patients who achieved clinical response, clinical remission, mucosal healing and endoscopic remission at Week 8 vs. those who did not achieve these endpoints (Table). Logistic regression results showed that PMS at Weeks 2 and 4, and CRP levels at Week 4, were significantly (
Conclusion
These post hoc analyses showed that PMS as early as Week 2 and CRP levels as early as Week 4 may be predictors of improved clinical and endoscopic outcomes in patients with moderately to severely active UC treated with tofacitinib 10 mg BID. These results are in alignment with the findings reported from the Phase 2 trial.1
Dubinsky MC
Sandborn WJ


