P375 Izencitinib induction treatment in patients with moderately-to-severely-active Crohn’s Disease: A phase 2 double-blind, randomized, placebo-controlled study
Schreiber, S.(1);Reinisch, W.(2);Nguyen, D.(3);Guerin, T.(4);Kierkus, J.(5);Rozpondek, P.(6);Bourdet, D.(7);Abhyankar, B.(8)*;Peyrin-Biroulet, L.(9);
(1)University Hospital Schleswig-Holstein- Kiel University- Kiel, Department of Internal Medicine I, Kiel, Germany;(2)Medical University of Vienna, Department Internal Medicine III, Vienna, Austria;(3)Former employee of Theravance Biopharma Inc, Research & Development, South San Francisco, United States;(4)Theravance Biopharma Ireland Ltd, Research & Development, Dublin, Ireland;(5)The Children’s Memorial Health Institute- Warsaw- Poland, Department of Gastroenterology, Warsaw, Poland;(6)Krakow Medical Center, Krakow Medical Center, Krakow, Poland;(7)Theravance Biopharma Inc., Research and Development, South San Francisco, United States;(8)Former Employee- Theravance Biopharma Ireland Ltd, Clinical Development, Dublin, Ireland;(9)Nancy University Hospital- University of Lorraine- Vandoeuvre-lès-Nancy- France., Department of Gastroenterology, Vandoeuvre-lès-Nancy, France;
Background
The unmet need in Crohn’s disease (CD) exists for new medications with an optimized risk-benefit ratio with avoidance of systemic side effects. This Phase 2 study evaluated the efficacy and safety of the oral, once-daily, gut-selective pan-Janus kinase inhibitor izencitinib as an induction therapy for CD.
Methods
This trial was a multicentre, randomized, double-blind, Phase 2 study of adults with moderately-to-severely active CD (>220 and ≤ 450 CDAI with SES-CD score of ≥6 [or ≥4 if isolated ileal disease]) who were corticosteroid-dependent or had demonstrated failure to aminosalicylates, corticosteroids, immunosuppressive agents, and/or biologic therapies. Patients were randomised 2:3:3 to receive placebo or izencitinib, 80, or 200 mg once daily for 12 weeks.
The primary endpoint was change in CDAI score from baseline to week 12 and was analysed using a mixed-model repeated measures analysis. Change from baseline in SES-CD was analysed with an analysis of covariance model and binary endpoints were analyzed using a stratified Cochran-Mantel-Haenszel chi-square test.
Results
159 and 145 patients were included in the safety and modified ITT populations, respectively.
At week 12, the LS mean change in CDAI was -105.6 (SE: 12.7) and -117.9 (12.4) points for patients randomized to izencitinib 80 and 200 mg, respectively, vs -104.86 (SE: 15.496) for those randomized to placebo. There were no improvements observed in change from baseline in SES-CD. There were non-significant numerical improvements in clinical and endoscopic response between placebo and the highest dose 200mg. No improvements were observed in CDAI-based clinical remission or PRO2 (SF/AP)-based remission at any dose of izencitinib relative to placebo.
As expected, plasma izencitinib exposure was low. Serious adverse events (AEs; n = 13) were well balanced among the treatment groups and not considered related to study treatment; no deaths were observed.
Exacerbation of Crohn’s Disease, headache, rash, abdominal pain and nausea were the most common treatment-emergent AEs observed with izencitinib treatment. No clinically important changes in laboratory values, electrocardiography parameters, or vital signs were noted in patients treated with izencitinib compared with placebo, and no drug-related AEs of special interest were observed.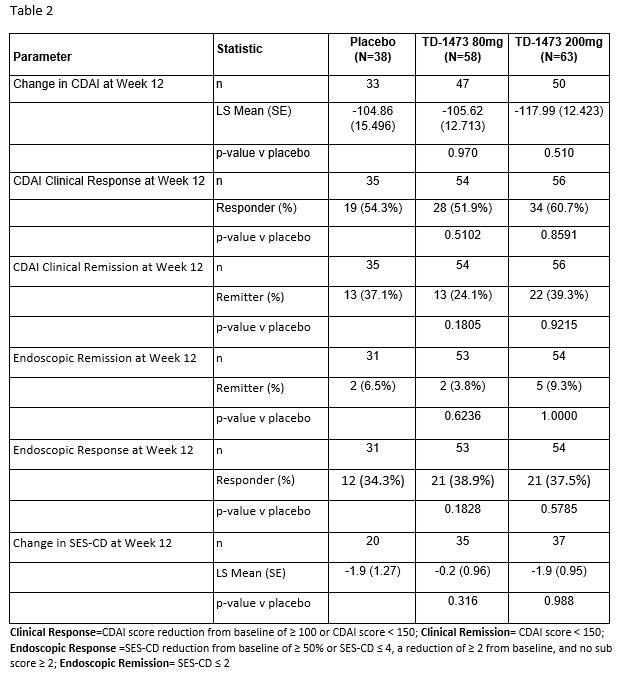
Conclusion
In patients with moderately-to-severely active Crohn’s Disease, izencitinib treatment for 12 weeks did not demonstrate a statistically significant reduction in CDAI or endoscopic severity. Izencitinib at both doses was well tolerated without a new safety signal.


