P377 Inadequate response with advanced therapies in real-world patients with Ulcerative Colitis – Results of a German claims database study
Bokemeyer, B.(1);Picker, N.(2);Wilke, T.(3);Rosin, L.(4);Patel, H.(5);
(1)Interdisziplinäres Crohn Colitis Center Minden und Medizinische Klinik I- Universitätsklinik Schleswig-Holstein- Campus Kiel, Gastroenterologische Praxis Minden, Minden, Germany;(2)Ingress Health, HWM GmbH, Wismar, Germany;(3)IPAM, e.V., Wismar, Germany;(4)Galapagos, Biopharma Deutschland GmbH, München, Germany;(5)Galapagos, Nv, Mechelen, Belgium
Background
Therapeutic management of Ulcerative Colitis (UC) is challenging, and clinicians are often obliged to attempt a variety of therapies in sequence until an adequate clinical response is achieved. However, real-world data regarding response rates in UC treatment are rare, particularly for later lines of therapy. Thus, this study aimed to investigate the incidence of inadequate response to advanced therapies in patients with UC.
Methods
This retrospective study was based on claims data from a regional German sickness fund covering the period from 01/2014-06/2019. Patients were included if they had at least two outpatient diagnoses in two different quarters or one inpatient diagnosis of UC (ICD-10: K51) and started a newly introduced advanced therapy (adalimumab, golimumab, infliximab, tofacitinib, vedolizumab) in 01/2015-06/2019. Patients were followed from treatment initiation (index date) until the end of the study period or loss to follow-up (median = 23.4 months).
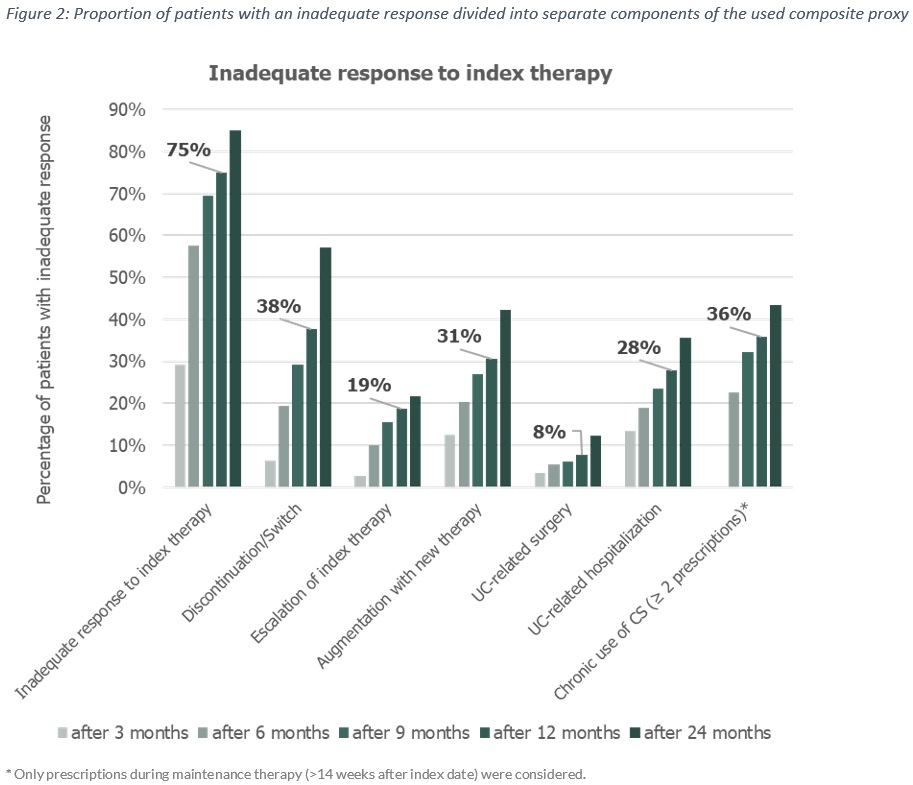
Proxies of inadequate response included: discontinuation (a supply gap of >60 days), switch, escalation (as dose increase exceeding 1.5 times the recommended maintenance dose), augmentation with 5-ASA, corticosteroid (CS) dependency (two CS prescriptions were observed starting more than 14 weeks after the index date), UC-related hospitalization, or UC-related surgery. CS dependency and treatment escalation were only assessed in the maintenance phase. Inadequate response in the analyzed sample was evaluated by means of Kaplan-Meier survival analysis.
Results
Among 574 UC patients (median age: 39 years; female: 53.5%), in whom an advanced therapy was initiated, 458 (79.8%) received an anti-TNF therapy, 113 (19.7%) vedolizumab and 3 (0.5%) tofacitinib. According to the available baseline period, 72 (12.4%) patients were identified as biologic-experienced. Most patients received CS (86.4%) and/or 5-aminosalicylic acids (81.7%) in the 12-month pre-index period. The median time to inadequate response to the initiated advanced therapy was 4.8 months (IQR: 2.6-11.9; Figure 1) with an inadequate response over 12 months in 75% (Figure 2). There was no significant difference in median time to inadequate response between biologic-naïve and biologic-experienced patients (4.9 vs. 4.7 months; p-value = 0.285). During the observable follow-up period, 172 (61%) patients switched from their index agent to another advanced therapy.
Conclusion
From the real-world settings in Germany, we found an inadequate response in UC-patients starting an advanced therapy in 75% of patients over 12 months. There is a need for more effective therapies among these patients.


