P378 Pharmacokinetics of Vedolizumab and Ustekinumab in pregnant women with Inflammatory Bowel Disease and their infants exposed in-utero
Prentice, R.(1)*;Flanagan, E.(2);Wright, E.K.(2);Gibson, P.R.(3);Rosella, S.(3);Rosella, O.(3);Goldberg, R.(1);Prideaux, L.(4);Kiburg, K.(5);Ross, A.L.(2);Burns, M.(1);Bell, S.J.(4);
(1)Monash Health, Gastroenterology, Melbourne, Australia;(2)St Vincent's Hospital Melbourne, Gastroenterology, Melbourne, Australia;(3)Monash University, Department of Medicine, Melbourne, Australia;(4)Monash Health, Gastroenterology, Clayton, Australia;(5)University of Melbourne, Department of Medicine, Melbourne, Australia; Pregnancy in Crohn's and Colitis: Observations Levels and Outcomes Extension (PICCOLO-X) Study Group
Background
The impact of pregnancy on vedolizumab (VDZ) and ustekinumab (UST) pharmacokinetics is not well defined. Similarly, the time to infant clearance and outcomes following in-utero exposure require elucidation. We aim to define the stability of UST and VDZ levels in pregnancy, placental drug transfer, and the time to and factors influencing infant drug clearance.
Methods
This multicentre prospective cohort study recruited women with IBD who were pregnant or planning pregnancy and receiving VDZ or UST. Trough drug levels, and clinical and biochemical data were documented pre-conception and in each trimester (T) of pregnancy. Maternal and infant drug levels were obtained at delivery and repeated in infants at intervals until clearance. Infant outcomes were assessed to 2 years of age. Drug levels were measured by ELISA (Theradiag) with a lower limit of detection of 0.25ug/mL (VDZ) and 0.4ug/mL (UST).
Results
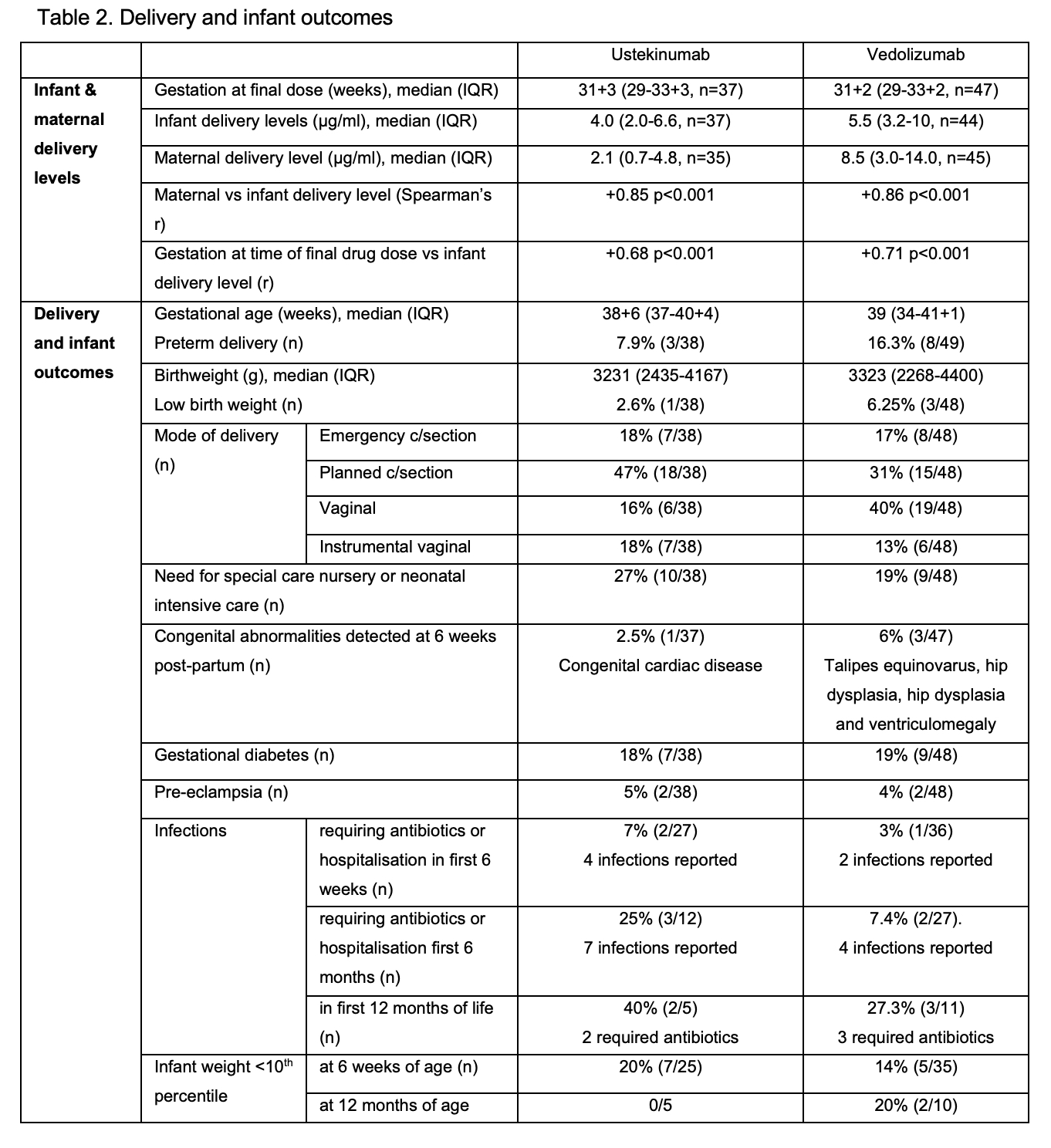
97 women aged median 31 (IQR 29-34) y with 54 receiving VDZ (41,17 with Crohn’s disease respectively) were included. Clinical and biochemical disease remission was maintained in the majority (Table 1). Median VDZ levels decreased by 42% from T1 to T3 (p<0.001; Skilling’s Mack) without a corresponding increase in faecal calprotectin (p=0.65; Wilcoxon signed-rank), whilst UST levels were stable over the course of pregnancy (p=0.14) (Fig 1). 82% (VDZ) and 78% (UST) of participants continued drug into T3. Favourable delivery and infant outcomes were observed (Table 2). Infant:maternal drug level ratio was 1.74 (1.24-3.5, n=35) for UST and 0.71 (0.52-0.91, n=44) for VDZ. Delivery levels in infants correlated positively with concomitant maternal levels and gestation at time of final drug dose (Table 2). 67% UST and 90% VDZ exposed infants cleared drug by 15 weeks (p=0.06; two sided test of proportions), with a time to clearance of 13 (9-22) weeks for UST and 11 (8-13) weeks for VDZ (p=0.11; non-parametric comparison of medians). Time to clearance correlated positively with infant delivery level (Table 2). There was no difference in the time to infant clearance between those receiving their last dose in T2 vs T3 (UST p=0.45 or VDZ p=0.44).

Conclusion
UST levels are stable over the course of pregnancy. VDZ levels fall but without a parallel increase in biochemical disease activity. Proactive dose adjustment and level monitoring during pregnancy is therefore not necessary. Differences in placental transfer of UST and VDZ were confirmed, with the infant:maternal drug level ratio at delivery lower for VDZ than UST. Both are cleared prior to 15 weeks in the majority, but the higher placental transfer of UST results in numerically fewer exposed infants clearing drug by this timepoint. No concerning infant outcomes were identified in this cohort.


