P383 Real-life experience of the efficacy, safety and pharmacokinetic data of switching from intravenous to subcutaneous infliximab in inactive inflammatory bowel disease patients. Results from the ENEIDA registry.
Iborra Colomino, M.I.(1)*;Garrido Marín, A.(1);Caballol Oliva, B.(2);Huguet Malavés, J.M.(3);Arias García, L.(4);Mesonero Gismero, F.(5);Fernández Prada, S.J.(6);Boscá Watts, M.M.(7);Ponferrada Díaz, Á.(8);Calvet Calvo, X.(9);Gutiérrez Casbas, A.(10); Ordás Jiménez, I.(2);Ruiz Sanchez, L.(3);Sicilia Aladren, B.(4);Garcia de la Filia, I.(5);Domènech Morral, E.(11);Nos Mateu, P.(1);
(1)Hospital Universitario y Politécnico la Fe de Valencia, Gastroenterology, Valencia, Spain;(2)Hospital Clínic de Barcelona, Gastroenterology, Barcelona, Spain;(3)Consorcio Hospital General Universitario de Valencia, Gastroenterology, Valencia, Spain;(4)Hospital Universitario de Burgos, Gastroenterology, Burgos, Spain;(5)Hospital Universitario Ramón y Cajal, Gastroenterology, Madrid, Spain;(6)Hospital Universitario Río Hortega de Valladolid, Gastroenterology, Valladolid, Spain;(7)Hospital Clínico Universitario de Valencia, Gastroenterology, Valencia, Spain;(8)Hospital Universitario Infanta Leonor Madrid, Gastroenterology, Madrid, Spain;(9)Hospital Universitari Parc Taulí, Gastroenterology, Sabadell, Spain;(10)Hospital General Universitario de Alicante Doctor Balmis, Gastroenterology, Alicante, Spain;(11)Hospital Universitari Germans Trias i Pujol, Gastroenterology, Badalona, Spain;
Background
Recently, a subcutaneous formulation of biosimilar infliximab (CT-P13) (SC-IFX) has been approved for inflammatory bowel disease (IBD). The aims of this study were to evaluate efficacy, safety, pharmacokinetics and patient experience following a switching to SC-IFX in patients who are in clinical remission on IV-IFX maintenance treatment.
Methods
Multicentre, descriptive, and observational study including Crohn’s disease (CD) and ulcerative colitis (UC) patients who were going to be changed from IV-IFX to SC-IFX on the ENEIDA registry (a large, prospectively maintained database of the Spanish Working Group in IBD–GETECCU). All patients were on clinical and biological remission at least 24 weeks before changing. Demographic and disease data, clinical activity (Harvey-Bradshaw index for CD and mayo index for UC), analytical data (C reactive protein (CRP) and fecal calprotectin (FC), as well as trough levels were collected at baseline, at 12 and 24 weeks.
Results
One hundred and fifty-five patients were included: 54 UC (35%) and 91 (65%) CD; 44% women and 56% men; age 45.5 years (32-55). IV-IFX was mainly administered due to active disease (72%) and perianal disease (7%) and during 32 months [range 14-56]. Pre-switch, 78 (50.3%) were on 8-weekly dosing of IV-IFX, 77 (49.7%) were with intensification dose and the half (50.3%) were on concomitant immunomodulatory therapy. SC-IFX was mainly switching by COVID-19 pandemic (60%), to increase through levels (15%) or patient request (25%). The majority of patients (140, 90%) remained with standard dose, 8 (5%) required dose intensification (120 mg weekly in 4 and 240 mg every 2 weeks in 4) and 7 (4.5%) had successful de-escalation (120 mg every 3 weeks in 4 and 120 mg every 4 weeks in 3). Clinical indices, CRP levels and FC remained unchanged (Figure). 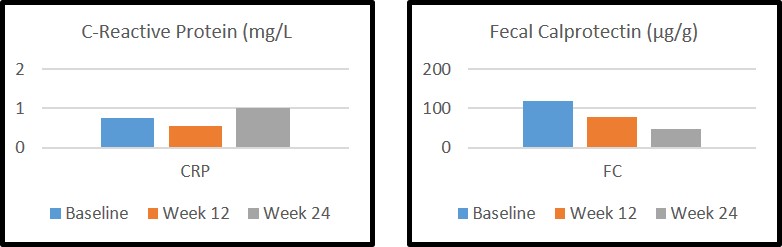 Median SC-IFX levels significantly increased from baseline of 4.5 μg/dl [range 2.6-9.2] to 14 μg/dl [range 9.5-16.2] at week 12 and 13.2 μg/dl [range 10.4-19.7] at week 24. No factors (immunossupresor, body mass index, disease location) were associated with the increase of IFX trough levels. During 24 weeks of follow-up, 16 of the 78 patients (20.5%) stopped immunosuppressant treatment. The adverse events were recorded in 9 patients (5.8%), 4 (2.6%) were hospitalized and 4 (2.6%) had surgery (one of them for perianal disease). Nine patients (5.8%) stopped SC-IFX (1 primary failure, 2 loss of response, 4 adverse events, 1 voluntarily, and 1 surgery).
Median SC-IFX levels significantly increased from baseline of 4.5 μg/dl [range 2.6-9.2] to 14 μg/dl [range 9.5-16.2] at week 12 and 13.2 μg/dl [range 10.4-19.7] at week 24. No factors (immunossupresor, body mass index, disease location) were associated with the increase of IFX trough levels. During 24 weeks of follow-up, 16 of the 78 patients (20.5%) stopped immunosuppressant treatment. The adverse events were recorded in 9 patients (5.8%), 4 (2.6%) were hospitalized and 4 (2.6%) had surgery (one of them for perianal disease). Nine patients (5.8%) stopped SC-IFX (1 primary failure, 2 loss of response, 4 adverse events, 1 voluntarily, and 1 surgery).
Conclusion
The switch from IV to SC IFX maintains clinical remission safely in IBD patients, offers higher drug levels and a good patient acceptance. However, the significance of higher drug levels with SC-IFX requires further exploration.


