P388 Agreement between locally and centrally reviewed Mayo endoscopic subscores in the UNIFI study of ustekinumab in patients with Ulcerative Colitis
Mishkin, D.(1);Marano, C.(2);Simi, A.(2);Lynch, J.(2);Noonan, L.(2);Zhang, H.(2);Sands, B.E.(3);Reinisch, W.(4);
(1)Atrius Health, Gastroenterology, Boston, United States;(2)Janssen Research & Development- LLC, Immunology, Spring House, United States;(3)Mount Sinai School of Medicine, Henry D. Janowitz Division of Gastroenterology, New York, United States;(4)Medical University of Vienna, Gastroenterology, Vienna, Austria; UNIFI
Background
In the UNIFI clinical study of ustekinumab (UST) in ulcerative colitis (UC), an endoscopic subscore (ES) was assigned by a local endoscopist, and the video was reviewed by central readers who provided an independent ES. Here, we determined ES agreement between local and central readers.
Methods
UNIFI (NCT02407236) was a placebo-controlled double-blind trial consisting of randomized induction and maintenance studies.1
Endoscopies were performed at screening (I-0), induction Wk 8 (I-8), and maintenance Wk 44 (M-44). Mayo ESs (range 0-3) were assigned by local endoscopists and a central reader reviewing the endoscopy video. The local reader was blinded to treatment, and the central reader was blinded to local ESs, treatment, and visit. If local and central readers assigned the same ES, that was the final ES (FES). If local and central reader ESs did not agree, videos were reviewed by an adjudicator who was blinded to the two previous ESs. If the adjudicator assigned the same ES as the local or central reader, that was the FES. If not, the FES was the median of the 3 ESs.
Results
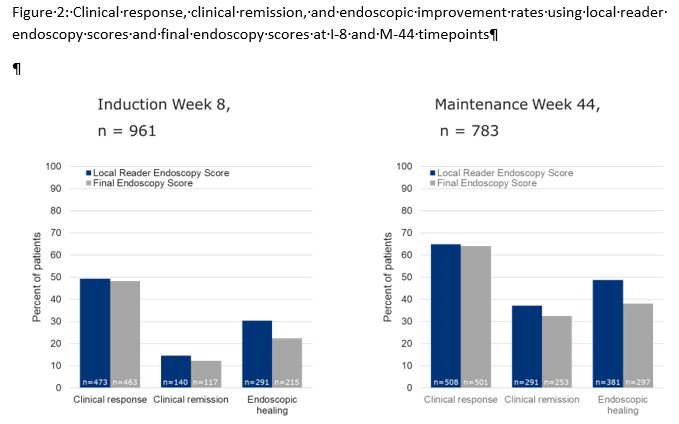
Agreement between individual local and central reader ESs was fair (κ=0.25-0.33, Figure 1) and was less than the agreement between central reader and adjudicator (κ=0.55–0.56) at all timepoints. However, at I-0, when determining study entry eligibility (ES≥2), local and central reader agreed more than 85% of the time.
At I-0, 98.2% of 1051 endoscopies with FES 2/3 were also scored 2/3 by local ES, while for the 104 with FES 0/1, 49% had a local ES 2/3. At I-8 and M-44 (primary endpoint timepoints), the local ES and FES agreed 87% (785/899) and 80% (497/625), respectively. The proportions of subjects with clinical remission and endoscopic improvement were slightly higher when using the local ES compared to the FES (Figure 2), whereas clinical response rates when using the local ES were similar to FES. Despite these findings, treatment differences between UST and placebo were generally similar when local ES and FES were used to calculate clinical remission at I-8 and M-88.
Approximately 90% of endoscopies had an image quality considered “optimal” or “readable with certainty but with minor technical difficulties” by central readers. Overall, image adequacy did not impact agreement between readers.
Conclusion
Even though agreement between local and central readers was fair and less than that between the central reader and adjudicator, the overall impact on primary and secondary study endpoints was minimal. Overall, the use of both local and central ES to determine the FES yielded a robust and reliable measure for study endpoint determination, however, efforts facilitating reproducible endoscopic assessments in UC are warranted.