P400 Identifying Nutritional targets in Crohn’s disease: INTICO1: a time-limited trial of exclusive enteral nutrition during adult CD remission
McDonnell, M.(1,2)*;Westoby, C.(3);Sartain , S.(1);Giannasca, P.(4);Corthésy, J.(5);Grzywinski, Y.(6);Frézal, A.(5);Capt, P.(5);Cummings, F.(1);Wootton, S.(7);
(1)University Hospital Southampton, Gastroenterology, Southampton, United Kingdom;(2)University of Southampton, Faculty of Medicine, Southampton, United Kingdom;(3)University Hospital Southampton, Department of Nutrition and Dietetics, Southampton, United Kingdom;(4)Nestle Health Science, Nestle Research, Cambridge, United States;(5)EPFL Innovation Park, Nestlé Research- Société des Produits Nestlé S.A, Lausanne, Switzerland;(6)EPFL Innovation Park, Nestlé Research- Société des Produits Nestlé S.A, Lasuanne, Switzerland;(7)University Hospital Southampton, Human Nutrition, Southampton, United Kingdom;
Background
During clinical remission in adult Crohn’s disease (CD), impaired health reacted quality of life and fatigue is common, and the nutritional state may be affected by factors such as restrictive eating and altered anatomy from previous inflammation or surgeries. Adults with CD in clinical remission do not routinely undergo nutritional assessments or intervention. The INTICO1 study used a time-limited trial of exclusive enteral nutrition (EEN) to expose and explore nutritionally sensitive aspects of in adults with CD during remission. The extent to which the nutritional state relates to the quality of CD remission and the potential role for nutritional therapies is neither well described nor understood.
Methods
This was a single arm longitudinal observation study with a dietary intervention. 24 adults in CD remission (Harvey Bradshaw <5, faecal calprotectin <250) underwent assessment of nutritional state at 3 time points; after habitual diet (assessment 1), after 7 days of polymeric EEN (assessment 2) and after a 2-week gradual return to habitual diet (assessment3). Assessments comprised food diary analysis, anthropometry, bio-electrical impedance analysis, routine pathology measures of micronutrient concentration, and patient reported outcome measures (PROMs).
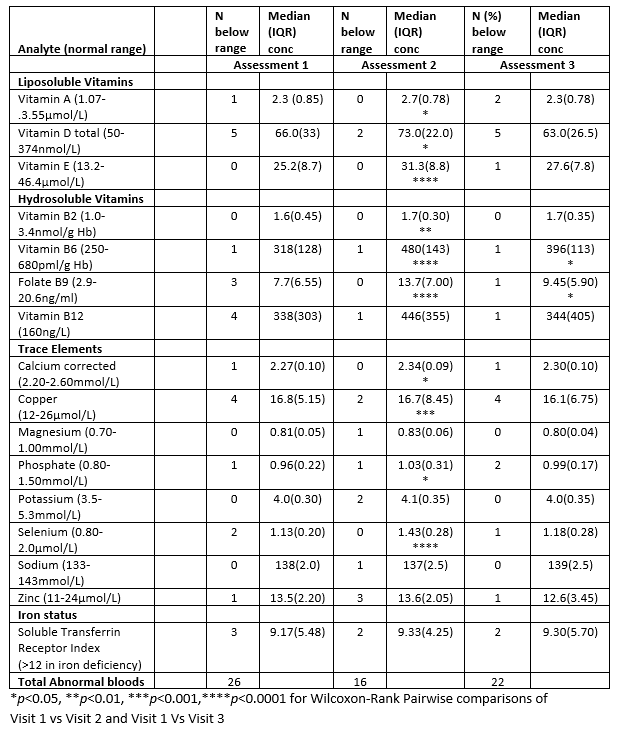
Results
Assessment 1 (habitual diet) identified individuals with multiple inadequacies of intake (7% (26/368)) micronutrients bloods below the normal laboratory range (see table for analytes) reduced lean mass, low phase angle (PhA) and excessive fatigue (reduced SF-36 vitality). At assessment 2 (post EEN), subjects had fewer deficiencies 4% (16/368), with significant increases seen in Vitamins A, D, E, B2, B6, B9 and the minerals Ca, Cu, Po4 and Se. At visit 3 (return to free diet), micronutrient concentrations reverted toward assessment 1 level, although B6 and B9 remained significantly higher (pairwise comparison visit 1 vs visit 3). BIA measures of nutritional status (PhA) and the SF36 physical function score improved 1 and 2 (5.2 vs 5.4 p<0.01 and 95 v100 p<0.05) and returned to baseline levels on return to free diet (Assessment 3).
Conclusion
A time-limited trial (7 days) of EEN, given in this setting of adults in CD remission led to changes in micronutrient biochemistry, objective improvement in the bioelectrical properties of the body (increased phase angle) and an improvement in fatigue among the subjects as captured by the SF36-PF. The study suggests that impaired nutritional state and excessive fatigue is likely to be prevalent among adults with CD during remission and may be sensitive to nutritional intervention.