P407 Efficacy and safety of etrasimod in subjects with moderately to severely active isolated proctitis: a subgroup analysis of the phase 3 ELEVATE UC 52 and ELEVATE UC 12 trials
Peyrin-Biroulet, L.(1,2)*;Dubinsky, M.C.(3);Sands, B.E.(4);Panés, J.(5);Schreiber, S.(6);Reinisch, W.(7);Feagan, B.G.(8);Danese, S.(9);Yarur, A.(10);D’Haens, G.(11);Goetsch, M.(12);Wosik, K.(13);Wu, J.(14);Modesto, I.(15);McDonnell, A.(16);Bartolome, L.(17);Rabbat, C.J.(18);Vermeire, S.(19);
(1)University of Lorraine- Inserm- NGERE, Gastroenterology, Nancy, France;(2)Groupe Hospitalier privé Ambroise Paré - Hartmann, Paris IBD Center, Neuilly sur Seine, France;(3)Feinstein IBD Center- Icahn School of Medicine, Gastroenterology, New York- New York, United States;(4)Icahn School of Medicine at Mount Sinai, Dr. Henry D. Janowitz Division of Gastroenterology, New York- New York, United States;(5)Hospital Clínic de Barcelona- IDIBAPS- CIBERehd, Gastroenterology, Barcelona, Spain;(6)Kiel University, University Hospital Schleswig-Holstein- Department Internal Medicine I, Kiel, Germany;(7)Medical University of Vienna, Department of Internal Medicine III- Division Gastroenterology and Hepatology, Vienna, Austria;(8)University of Western Ontario/Alimentiv Inc, Gastroenterology, London- Ontario, Canada;(9)IRCCS Ospedale San Raffaele and University Vita-Salute San Raffaele, Gastroenterology, Milan, Italy;(10)Cedars-Sinai Medical Center, Gastroenterology, Los Angeles- California, United States;(11)University of Amsterdam, Gastroenterology, Amsterdam, The Netherlands;(12)Pfizer AG, Global Clinical Development, Zurich, Switzerland;(13)Pfizer Inc, Global Medical Affairs- Gastroenterology, Kirkland- Quebec, Canada;(14)Pfizer Inc, Global Product Development, Groton- Connecticut, United States;(15)Pfizer Inc, Global Medical Affairs- Gastroenterology, Madrid, Spain;(16)Pfizer- Ltd, Safety Risk, Walton Oaks- Scurry, United Kingdom;(17)Pfizer Inc, Global Health Economics & Outcomes Research- Inflammation & Immunology, New York- New York, United States;(18)Pfizer Inc, Global Medical Affairs- Gastroenterology, New York- New York, United States;(19)University Hospitals Leuven, Gastroenterology, Leuven, Belgium;
Background
Etrasimod is an investigational, once-daily, oral, selective sphingosine 1-phosphate receptor 1,4,5 (S1P1,4,5) modulator in development for the treatment of moderately to severely active ulcerative colitis (UC). Although ≈30% of UC patients initially present with isolated proctitis, most phase 3 trials of systemic therapies in UC excluded this subgroup. We report the efficacy and safety of etrasimod in subjects with isolated proctitis enrolled in the ELEVATE UC programme.
Methods
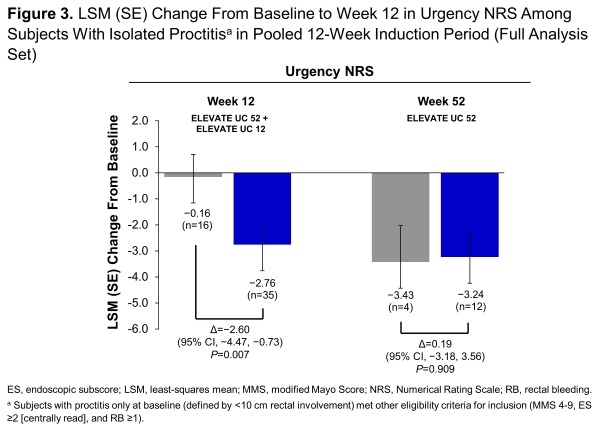
In ELEVATE UC 52 (NCT03945188) and ELEVATE UC 12 (NCT03996369), subjects (16-80 years) with moderately to severely active UC were randomised 2:1 to once-daily etrasimod 2 mg or placebo (PBO). ELEVATE UC 52 comprised a 12-week induction period followed by a 40-week maintenance period with a treat-through design. ELEVATE UC 12 comprised a 12-week induction period. Both trials allowed inclusion of subjects with isolated proctitis (<10 cm rectal involvement) provided they met all other eligibility criteria (enrolment capped at 15%). Predefined endpoints were assessed at week 12 (data pooled from both studies) and week 52 (UC 52 only). Sustained and corticosteroid (CS)-free clinical remission were assessed at week 52 (UC 52 only). Changes from baseline in rectal bleeding (RB) were assessed using pooled data through week 12, and UC 52 data from week 14 through week 52. Urgency Numeric Rating Scale (NRS) was assessed at baseline, week 12 (pooled), and week 52 (UC 52 only).
Results
This analysis included 64 pooled subjects with isolated proctitis at week 12 (42 etrasimod, 22 PBO) and 36 subjects from UC 52 at week 52 (27 etrasimod, 9 PBO). Greater proportions (P<0.05) of subjects randomised to etrasimod vs PBO achieved clinical remission, endoscopic improvement, symptomatic remission, and endoscopic improvement-histologic remission at week 12 and week 52 and clinical remission and symptomatic remission at week 52 (Figure 1). Differences were also observed in sustained clinical remission and 12-week CS-free clinical remission at week 52. Etrasimod demonstrated numerical decreases in RB subscores as early as week 2 through week 52 (Figure 2). Improvement (P<0.05) in urgency NRS were observed at week 12 (Figure 3). Safety was consistent with results from the overall trial populations.

Conclusion
Efficacy and safety of etrasimod in subjects with isolated proctitis was consistent with the overall population of the ELEVATE programme.1 The analysis was performed on a small dataset and should be interpreted with caution but informs on the efficacy of etrasimod in a subpopulation of UC subjects commonly excluded from drug development trials.
Reference: 1. Sandborn WJ, et al. Presented at: DDW 2022; May 24, 2022. Abstract 968a.


