P409 Endoscopic and histological activity is associated with non-response to biologic therapy in ulcerative colitis
S. WANN1, R. Norris1, R. Williams2, C. Basnayake1, M. Lust1, E. Wright1, M. Kamm1, W. Connell1, A. Thompson1, N. Ding1
1Gastroenterology, St Vincent’s Hospital Melbourne, Melbourne, Australia, 2Anatomical Pathology, St Vincent’s Hospital Melbourne, Melbourne, Australia
Background
Ulcerative colitis (UC) is a chronic inflammatory bowel disease, characterised by a relapsing and remitting course. Biologic therapy in UC has a 30% rate of primary non-response (PNR) and a further 5% loss of response per year. Prognostic indicators of non-response include younger age and longer disease duration. Emerging evidence suggests that endoscopic and histological activity is associated with adverse outcomes such as clinical relapse and colectomy. However, the role of endoscopic or histological disease activity in predicting non-response to biologic therapy is unclear. We aim to investigate the association of endoscopic and histological activity with non-response to biologic therapy in UC.
Methods
Patients with UC treated with biologic therapy between 2014 and 2019 were studied. Baseline endoscopic disease activity was assessed using the Mayo endoscopic subscore from endoscopy performed prior to commencing biologic therapy. Patients who underwent colonoscopy 3–9 months after commencing biologic therapy were selected for histopathological review. Sigmoid biopsies were evaluated by an expert gastrointestinal pathologist. Histological parameters were scored on a 4-point scale, with blinding to clinical and endoscopic outcomes.
Results
Sixty-six patients were followed for a median of 16 months (IQR 6–30), 12 (18%) of whom did not respond to biologic therapy initially and 3 (6%) of whom lost response secondarily. Baseline endoscopic activity was associated with non-response (OR 6.1, 95% confidence interval 1.3 – 29.4, for every 1-point increase in the 4-point Mayo endoscopic subscore). 23 patients were identified for histopathological review. Four (17%) patients exhibited PNR. Baseline crypt atrophy was associated with PNR (
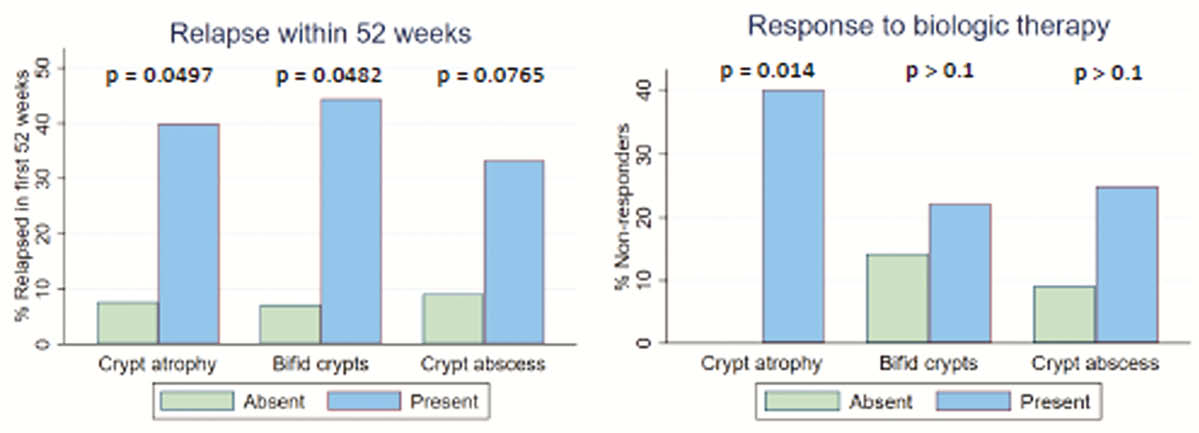
Conclusion
For patients with UC, endoscopic disease activity assessed prior to commencing biologic therapy has the potential to predict non-response to biologic therapy. In addition, specific histological parameters relating to crypt inflammation and crypt architecture may also predict non-response. Further prospective studies should be conducted to investigate the clinical utility of assessing endoscopic and histological disease activity prior to commencing biologic therapy.


