P410 Long-term persistence and safety of biological drugs in patients with Inflammatory Bowel Disease. Differences between women and men: SEXEII study of ENEIDA
Gargallo-Puyuelo, C.(1)*;Ricard, E.(2);Iborra, M.(3);Iglesias-Flores, E.(4);Vera Mendoza, I.(5);De Francisco García, R.(6);Calafat Sard, M.(7);Minguez, M.(8);Lopez- San Roman, A.(9);Taxonera, C.(10);Guardiola, J.(11);Barrio, J.(12);Laredo, V.(13);De Castro, L.(14);Gisbert, J.(15);García-Lopez, S.(16);García Planella, E.(17);Martín Arranz, D.(18);Calvet, X.(19);Merino, O.(20);Sierra, M.(21);Marquez, L.(22);Madero, L.(23);Varela, P.(24);Carpio, D.(25);Esteve, M.(26);Rivero, M.(27);Ramos , L.(28);Sicilia, B.(29);Lorente POyatos, R.(30);Marin, I.(31);Monfort, D.(32);Navarro, M.(33);VEga, P.(34);Hinojosa, J.(35);Tardillo, C.(36);García Sepulcre, M.F.(37);Barreiro, M.(38);Domenech, E.(7);Gomollón, F.(39);
(1)Hospital Clínico Universitario Lozano Blesa, Gastroenterology., Zaragoza, Spain;(2)H. Clínic Barcelona, Gastroenterology, Barcelona, Spain;(3)Hospital Universitario La Fe de Valencia, Gastroenterology, Valencia, Spain;(4)H. Reina Sofía, Gastroenterology, Cordoba, Spain;(5)H.U. Puerta de Hierro, Gastroenterology, Madrid, Spain;(6)Hospital Universitario Central de Asturias, Gastroenterology, Oviedo, Spain;(7)H.U. Germans Trias i Pujol, Gastroenterology, Barcelona, Spain;(8)H. Clínico de Valencia, Gastroenterology, Valencia, Spain;(9)H. Ramón y Cajal, Gastroenterology, Madrid, Spain;(10)Hospital Clínico San Carlos, Gastroenterology, Madrid, Spain;(11)H.U. Bellvitge, Gastroenterology, Bellvitge, Spain;(12)Hospital Rio Hortega, Gastroenterology, Valladolid, Spain;(13)Hospital Ernest LLuch, Gastroenterology, Calatayud, Spain;(14)Hospital Álvaro Cunqueiro, Gastroenterology, Vigo, Spain;(15)Hospital de La Princesa, Gastroenterology, Madrid, Spain;(16)Hospital Universitario Miguel SErvet, Gastroenterology, Zaragoza, Spain;(17)H. de la Sta Creu i Sant Pau, Gastroenterology, Barcelona, Spain;(18)H. La Paz, Gastroenterology, Madrid, Spain;(19)Hospital de Sabadell, Gastroenterology, Sabadell, Spain;(20)Hospital de Cruces, Gastroetnerology, Bilbao, Spain;(21)Complejo Asistencial Universitario de León, Gastroenterology, Leon, Spain;(22)H. Del Mar, Gastroenterology, Barcelona, Spain;(23)Hospital General Universitario de Alicante, Gastroenterology, Alicante, Spain;(24)H. Cabueñes, Gastroenterology, Gijon, Spain;(25)Complexo Hospitalario Universitario de Pontevedra, Gastroenterology, Pontevedra, Spain;(26)H. Mútua Terrassa, Gastroenterology, Barcelona, Spain;(27)H. U. Marques de Valdecilla, Gastroenterology, Santander, Spain;(28)H.U. Canarias, Gastroenterology, Tenerife, Spain;(29)Hospital Universitario de Burgos, Gastroenterology, Burgos, Spain;(30)H. General de Ciudad Real, Gastroenterology, Ciudad REal, Spain;(31)H.G.U. Gregorio Marañón, Gastroenterology, Madrid, Spain;(32)Consorci Sanitari de Terrassa, Gastroenterology, Terrasa, Spain;(33)H. Moisès Broggi, Gastroenterology, San JOan Despi, Spain;(34)Complexo H. Universitario de Ourense, Gastroenterology, Ourense, Spain;(35)H. Manises, Gastroenterology, Manises, Spain;(36)H. Nuestra Sra. de la Candelaria, Gastroenterology, Tenerife, Spain;(37)H.G.U. Elche, Gastroenterology, Elche, Spain;(38)H.U. de Santiago, Gastroenterology, Santiago de Compostolea, Spain;(39)Hospital Clínico Universtario Lozano Blesa, Gastroenterology, Zaragoza, Spain; on behalf of the ENEIDA Registry of GETECCU
Background
Female sex has been associated with a worse response to anti-TNF drugs and with discontinuation of these drugs in immune-mediated diseases. Data in Inflammatory Bowel Disease (IBD) are unclear. The aims of study are to assess possible differences in long-term treatment persistence and safety of biological drugs between women and men with IBD.
Methods
Multicenter observational study carried out with data from the ENEIDA registry. Patients diagnosed with Crohn's disease (CD) or ulcerative colitis (UC) who receive or have received biological drugs, and have had a minimum treatment follow-up of 6 months, were included. We evaluated the first biological treatment used in the patient.
Statistic analysis; Regression logistic models were used for safety evaluation. Kaplan-Meier curves, Log-Rank test and COX regression were used to treatment persistance.
Results
A total of 51,595 patients with IBD were evaluated [ 25,947 with CD (13238 men and 12709 women) and 25,648 with UC (13596 men and 12052 women)]. Mean follow up of 13 years.
Biologic use: 28.7% of the evaluated patients had been treated with at least one biologic drug. Biologics use in UC was less common in women than in men (15.5% vs. 17.2%, OR (95%CI): 0.88 (0.81-0.94), p= 0.001) and there were no differences between sexes in CD ( 45.7% in men, 44.7% in women). Infliximab (IFX) and adalimumab (ADA) were the most used drugs (in 8914 and 5269 patients, respectively).
Safety evaluation. Women suffered more frequently adverse effects to IFX and ADA than men, being the withdrawal of IFX and ADA due to adverse effects also significantly more frequent in women than in men. Figure 1.
Biological treatment persistence in patients with IBD was longer in men than in women [median 3.1 years vs. 2.3 years, p < 0.001]. Female sex was a risk factor of biologic discontinuation [adjusted hazard ratio [aHR] (95%CI): 1.20 (1.14-1.25), p<0.001]. Combotherapy with thiopurines and CD were protective factors for discontinuation [ combotherapy; aHR (95%CI): 0.46 ( 0.44-0.48), p<0.001; CD aHR (95%CI): 0.78 (0.74-0.82), p < 0.001]. In the analysis of each specific biological drug, female sex was identified as independent predictor for discontinuation of IFX [aHR (95%CI) 1.21; 1.13-1.30, p<0.001] and ADA [aHR (95%CI) 1.24; 1.14-1.35, p<0.001] in patients with CD and of IFX in patients with UC [aHR (95%CI): 1.16; 1.05-1.28, p=0.004]. 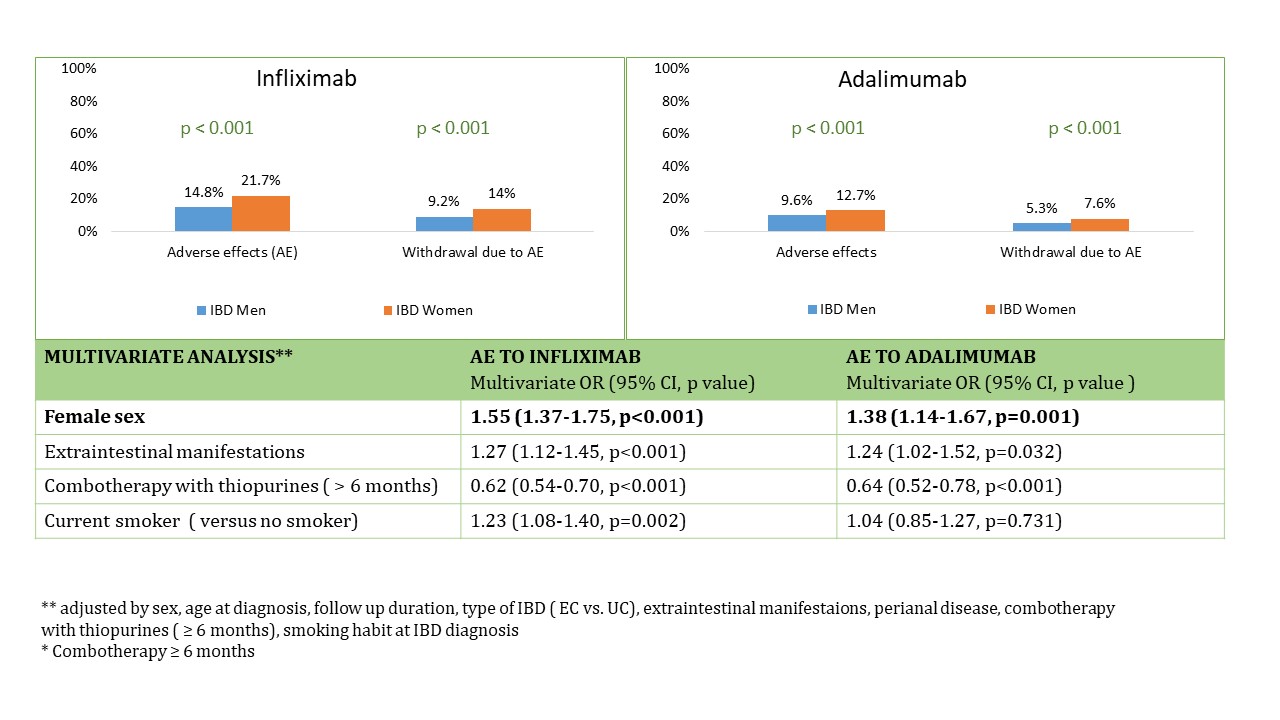
Conclusion
1.The use of biologics in ulcerative colitis seems to be higher in men than in women.
2. Female sex is an independent risk factor for the development of adverse effects to IFX and ADA and for the discontinuation of these drugs.
3. The long-term persistence of IFX and ADA (as first biological treatments) is low, being higher in men compared to women.


