P414 Comparative real-world effectiveness and persistence of vedolizumab versus anti-TNF therapy in biologic-naïve patients with Crohn´s disease with propensity score adjustment: two-year maintenance phase results from the prospective VEDO-IBD study
Bokemeyer, B.(1,2,3)*;Plachta-Danielzik, S.(3);Efken, P.(4);Mohl, W.(5);Hoffstadt, M.(6);Krause, T.(7);Schweitzer, A.(8);Schnoy, E.(9);Atreya, R.(10);Teich, N.(11);Trentmann, L.(12);Ehehalt, R.(13);Franzenburg, S.(3);Hartmann, P.(4);di Giuseppe, R.(3);Schreiber, S.(2,3);
(1)Interdisciplinary Crohn Colitis Centre Minden, Crohn Colitis Centre, Minden, Germany;(2)University Hospital Schleswig-Holstein- Campus Kiel, Clinic of General Internal Medicine I, Kiel, Germany;(3)Competence network IBD, Study department, Kiel, Germany;(4)Gastroenterology Practice Minden, Practice, Minden, Germany;(5)Center for Gastroenterology Saar MVZ, Practice, Saarbruecken, Germany;(6)Gastroenterology Practice Iserlohn, Practice, Iserlohn, Germany;(7)Gastroenterology Practice Kassel, Practice, Kassel, Germany;(8)Gastroenterology Practice Muenster, Practice, Muenster, Germany;(9)University Hospital Augsburg, III. medical clinic, Augsburg, Germany;(10)Friedrich-Alexander-University Erlangen-Nuernberg, Medical Clinic 1, Erlangen, Germany;(11)Gastroenterology Practice Leipzig, Practice, Leipzig, Germany;(12)Gastroenterology Practice Bremen, Practice, Bremen, Germany;(13)Gastroenterology Practice Heidelberg, Practice, Heidelberg, Germany;
Background
To gain insight into vedolizumab (VEDO) use as a first-line biologic in Crohn´s disease (CD), this comparative, two-arm prospective real-world-evidence (RWE) study with propensity score (PS) adjustment aimed to assess, within the maintenance phase, the 2-year comparative effectiveness and persistence of VEDO vs anti-TNF therapy in biologic-naïve CD patients.
Methods
Between 2017-2020, 1200 consecutively enrolled biologic-naïve and biologic-experienced patients with ulcerative colitis (UC) and CD were prospectively included in the VEDOIBD study from 45 IBD-experienced centres across Germany. 260 biologic-naïve CD patients starting a new therapy with VEDO or anti-TNF were included in this RWE comparison of VEDO vs anti-TNF. The Kaplan-Meier curve was used to summarize the treatment persistence from the start of therapy through week-104. The primary outcome was two-year clinical remission (HBI ≤ 4). Patients were analysed on a modified intent-to-treat basis (mITT; switchers considered as outcome failure). To reduce the effect of confounders, PS adjustment with inverse probability of treatment weighting (IPTW) was implemented. A weighted logistic regression was used to evaluate the effectiveness. The results were reported as odds ratio (OR) and 95% confidence interval (CI).
Results
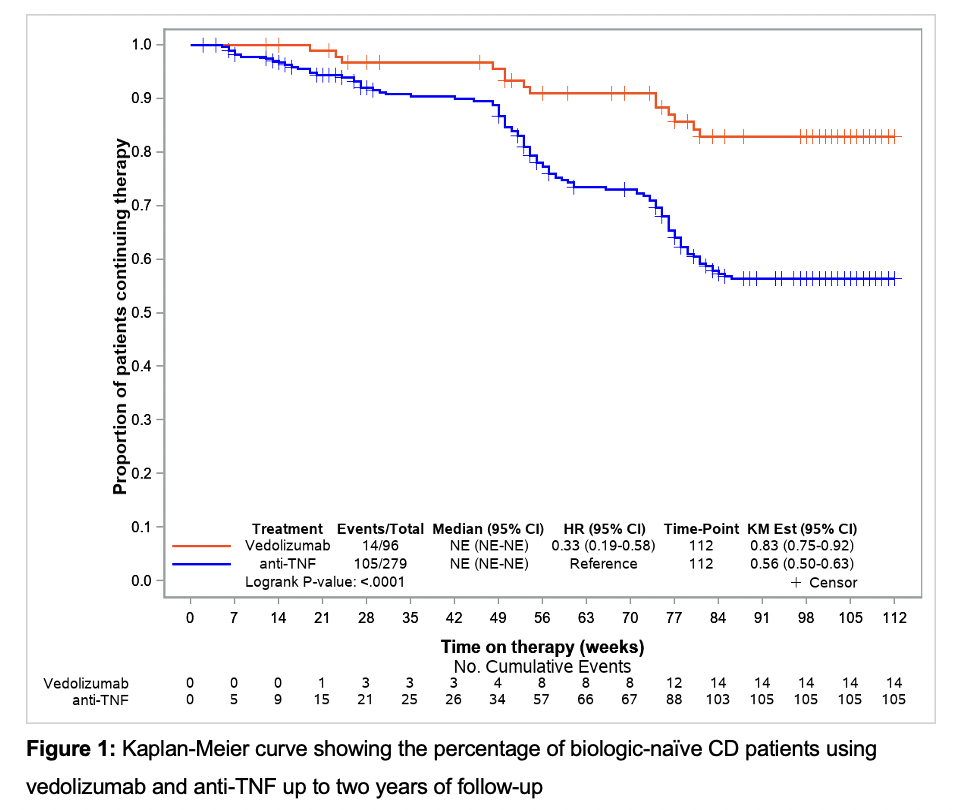
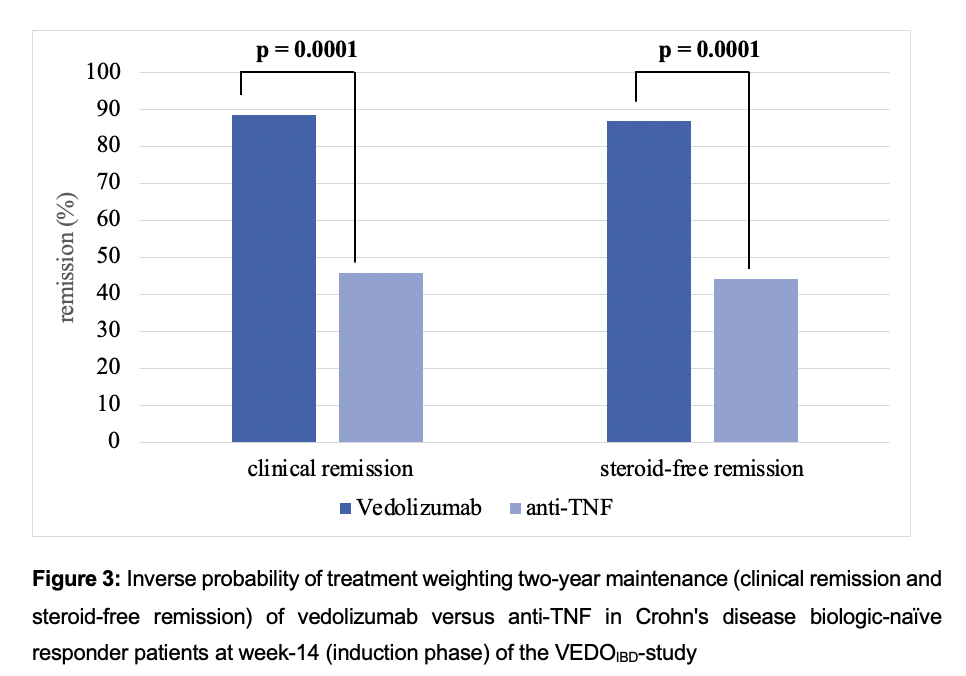
63 VEDO and 197 anti-TNF (ADA: 58.4%, IFX: 41.6%) biologic-naïve CD-patients were evaluated. Two years after treatment initiation approximately 83% of VEDO patients were still in continuous treatment vs only 56% of anti-TNF patients (Fig. 1). In the mITT analysis (Fig. 2), there was a significantly higher clinical remission rate with VEDO when compared to anti-TNF after two years (VEDO: 64.2% vs anti-TNF: 44.7%), and a higher steroid-free remission (VEDO: 62.5% vs anti-TNF: 41.6%). Both differences were statistically significant (p<0.05). Additionally (Fig. 3), using IPTW to examine the two-year maintenance effectiveness in week-14 induction phase responders, there was a significantly better response in terms of clinical remission for VEDO (88.6%) compared to anti-TNF (45.8%) (p=0.0001) and in steroid-free remission of 86.8% for VEDO compared to 44.1% for anti-TNF (p<0.001).
Conclusion
Compared to previous RCTs, this prospective two-year RWE study comparing VEDO with anti-TNF showed that, in biologic-naïve CD patients, remission rates at two years with VEDO were remarkably higher than with anti-TNF. Given the favourable side effect profile of VEDO, these findings may aid physicians’ decision-making on the choice of VEDO as a first-line biologic for CD.