P417 Frequent Early Nocebo Complaints but Maintained Clinical Efficacy, Biomarker and Therapeutic drug levels following mandatory Biosimilar Switch in Patients with Inflammatory Bowel disease: Preliminary data from a Prospective Observational Cohort Stud
Wetwittayakhlang, P.(1)*;Karkout, K.(2);Tselekouni, P.(1);Aljabri, R.(1);Bessissow, T.(1);Afif, W.(1);Wild, G.(1);Bitton, A.(1);LakatosPhD, P.L.(3,4);
(1)McGill University Health Centre, Division of Gastroenterology and Hepatology, Montreal, Canada;(2)McGill University Health Centre, Department of Internal Medicine, Montreal, Canada;(3)Mcgill University Health Center, IBD Centre, Montréal, Canada;(4)Semmelweis University, Department of Internal Medicine and Oncology, Budapest, Hungary;
Background
Current evidence suggests that biosimilar is safe and effective for patients with IBD. There is limited data on and nocebo effect after switching from originator to biosimilar. We aimed to report clinical efficacy, therapeutic drug monitoring, adverse events (AEs), and frequency of nocebo in IBD patients with biosimilar switches.
Methods
We performed a prospective observational study of 257 consecutive IBD patients who underwent biosimilar switch at McGill University Health Centre, Montreal, Canada, between November 2021 and October 2022. We excluded 74 patients due to missing follow-up data/delay in the switch. Patients underwent a mandatory switch from the originator (Remicade or Humira) to biosimilars due to a change of the reimbursement policy in Quebec. Clinical and biochemical data were captured at 8 weeks before switch, baseline (time of the switch), and 12 and 24 weeks after the switch. Clinical remission was defined as an HBI<5 for Crohn's disease (CD) or a partial Mayo<3 points for ulcerative colitis (UC). Biomarkers remission was defined as C-reactive protein (CRP<5mg/mL) and fecal calprotectin (FCAL)<250 mcg/g. Drug trough levels and anti-drug antibodies were measured at baseline and week 24 (therapeutic level was defined as infliximab level>3mcg/mL, adalimumab>5mcg/mL). AEs and nocebo effects were assessed.
Results
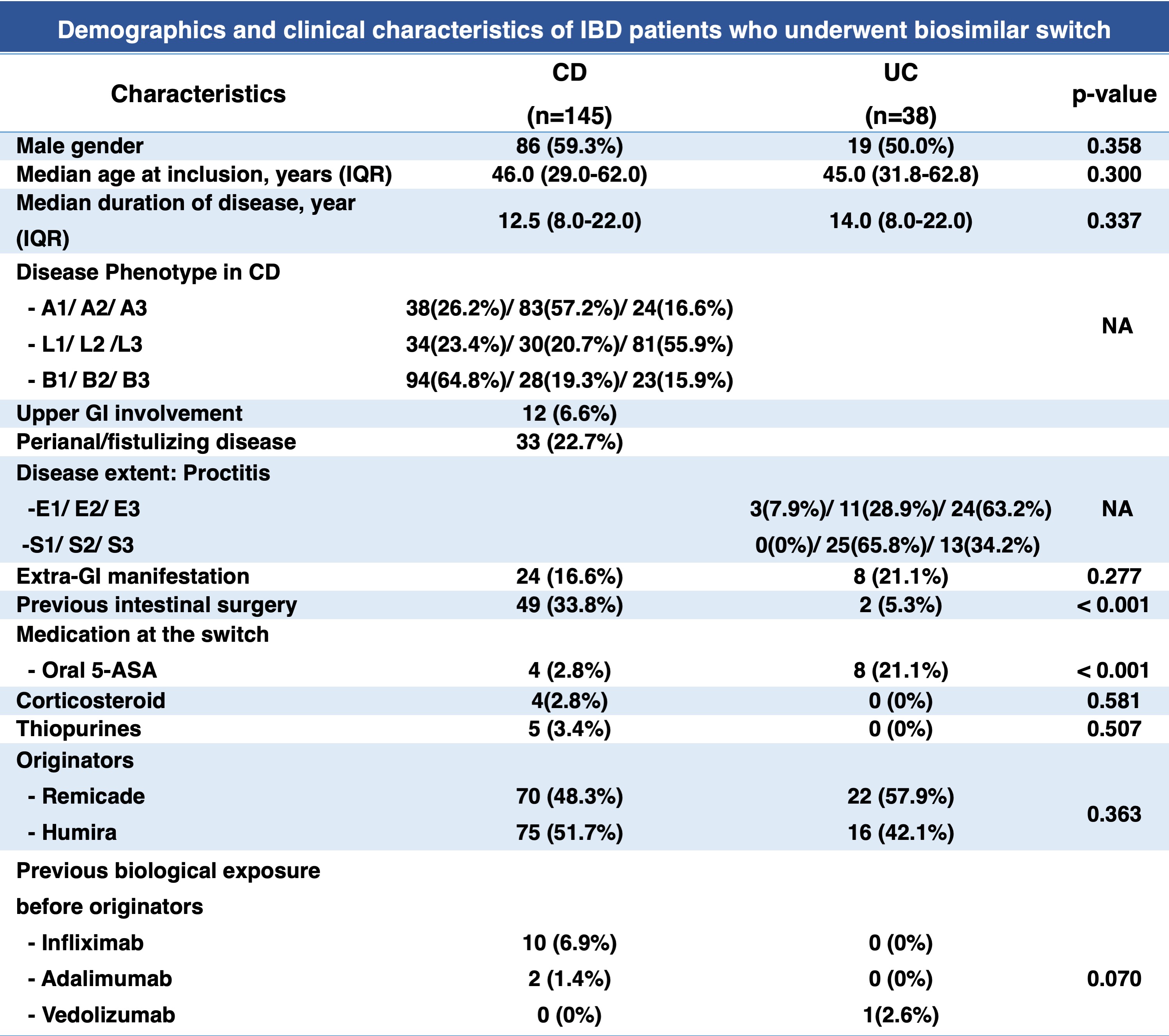
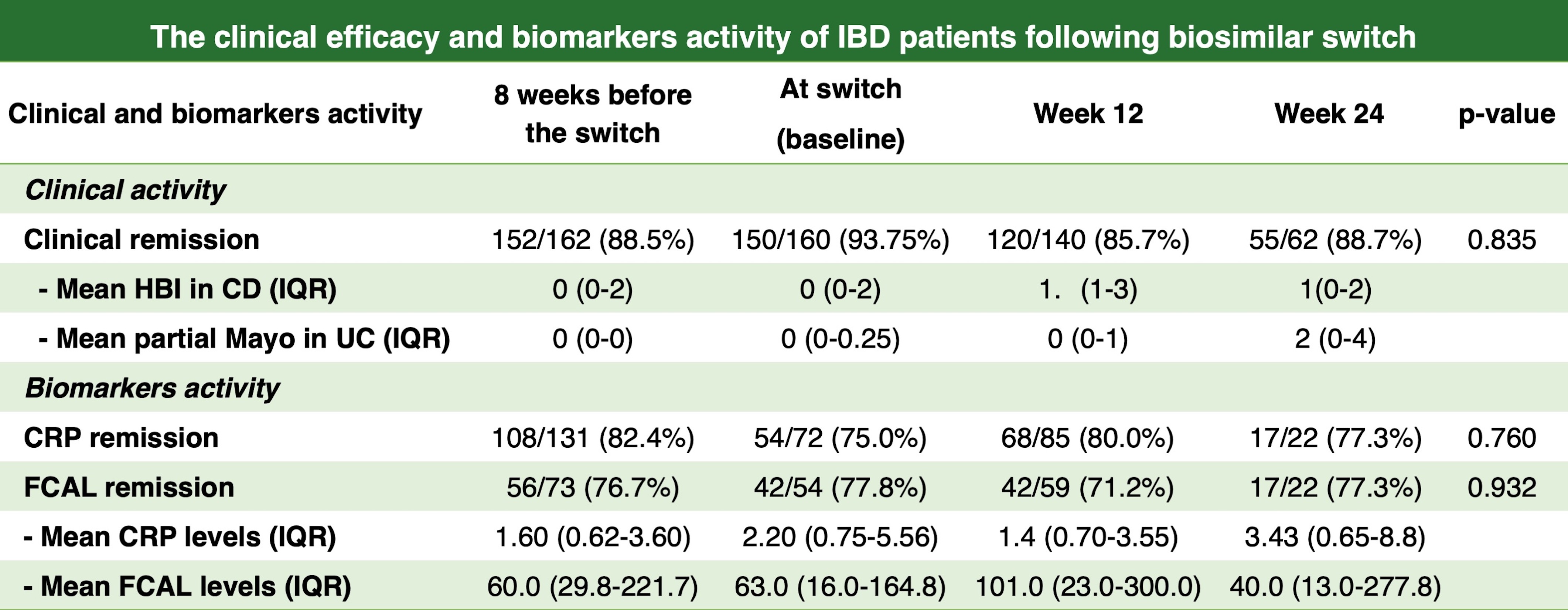
A total of 183 patients were included [79.2% were CD, male 57.4%, median age at inclusion 46 years (IQR 30-52), and median duration of disease 13 years (IQR8-22)]. There was no significant difference in the proportion of patients in clinical remission at week 8 before the switch(88.5%), baseline(93.8%), week 12(85.7%) and week 24(88.7%), p=0.835. Similarly, the proportion of patients remaining in biomarkers remission was not significantly different at week 8 before the switch, baseline, week 12 and week 24; CRP (82.4%/75.0%/80.0%/77.3%), p=0.760; FCAL (76.7%/77.8%/71.2/77.3%), p=0.932, as well as there was no significant difference in the proportion of patients maintaining the therapeutic level, (85.5%/82.2%/79.6%, p=0.853) and prevalence of positive anti-drug antibody at (10.5%/4.4%/11.8%, p=0.190) at 8 weeks before switch, baseline, and week 24, respectively. 14.5% of patients had subtherapeutic drug levels, 6% underwent dose optimization, and 4.4% had a loss of response/relapse. Nocebo was reported in 12% of patients (9.3% within the first 12 weeks and 2.7% within the first 24 weeks after the switch), 5.5% had drug AEs,and 3.8% discontinued biosimilars due to AEs.


Conclusion
Despite a significant number of early nocebo complaints within the first 3 months after the biosimilar switch, no significant changes were found in clinical efficacy, therapeutic drug trough level, and anti-drug antibodies.


